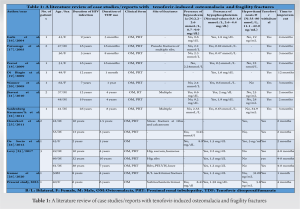
People living with HIV and AIDS taking a tenofovir-based HAART regimen are prone to fragility fracture which can be prevented by regular monitoring of bone health including serum vitamin D3 levels.
Dr. Karthick Rangasamy, Department of Orthopaedics, Postgraduate Institute of Medical Education and Research, Chandigarh, India. E-mail: drsrk05@gmail.com
Introduction: The population of people living with human immunodeficiency virus (HIV) and AIDS has increased and so is the incidence of fragility fractures in these patients. Multiple contributory factors are responsible for osteomalacia or osteoporosis in such patients such as a chronic inflammatory response to the HIV, highly active antiretroviral therapy (HAART) itself, and associated comorbidities. Tenofovir has also been reported to disrupt bone metabolism and causes fragility fractures.
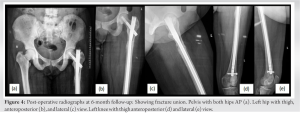
Case Report: A 40-year-old HIV-positive female came to us with pain in her left hip and was unable to bear weight. She had a history of trivial fall. The patient has been taking tenofovir-associated HAART regimen for the past 6 years and has been compliant. She was diagnosed with a left-side transverse subtrochanteric closed femur fracture. Closed reduction and internal fixation was done using a proximal femur intramedullary nail (PFNA). The latest follow-up shows fracture union and good functional outcomes after treating osteomalacia, and HAART changed to a non-tenofovir regimen later.
Conclusion: Patients with HIV infection are prone to fragility fractures and periodic monitoring of their BMD, serum calcium, and vitamin D3 levels should be done for prevention and early diagnosis. More vigilance in patients receiving a tenofovir-associated HAART regimen is needed. Appropriate medical treatment needs to be started once any abnormality in the bone metabolic parameters is detected, and drugs like tenofovir need to be changed as it causes osteomalacia.
Keywords: Human immunodeficiency virus, fracture, pathological, HAART, tenofovir, osteomalacia.
Highly active antiretroviral therapy (HAART) has significantly made the lives of people living with human immunodeficiency virus (PLHIV) better in a way that these individuals can now lead normal lives. However, due to the increased life span in these patients, there was a rise in the prevalence of various chronic age-related illnesses such as hypertension, diabetes, cardiovascular disease, and stroke [1]. Bone health was also shown to be affected in PLHIV compared to HIV-negative individuals, resulting in an increased prevalence of osteoporosis and fragility fractures in them [2]. Furthermore, the drugs used as part of HAART, especially tenofovir, have been shown to disrupt the balance between vitamin D and parathyroid hormone (PTH) causing osteoporosis and osteomalacia [3]. Hereby, we describe a case of tenofovir-induced osteomalacia resulting in fragility fracture in a middle-aged female receiving HAART for the past 6 years. We also present the results of the literature review on tenofovir-induced osteomalacia and fragility fractures. The consent for the use of images and clinical details for the publication was obtained from the patient.
A 40-year-old premenopausal HIV-positive female presented to the outpatient department with left hip pain for 2 days following a trivial fall while going to the bathroom in May 2021. She was unable to bear weight on the left lower limb after the fall. She was diagnosed with HIV infection during routine pre-natal blood investigations at the time of her second pregnancy in 2015. She was otherwise asymptomatic for the HIV infection and had a baseline CD4 count of 146/µL. Antiretroviral therapy (ART) was initiated which included lamivudine (300 mg), tenofovir (300 mg), and efavirenz (600 mg), a fixed-dose combination once daily at night. Cotrimoxazole (Sulphamethoxazole 800 mg and Trimethoprim 160 mg) prophylaxis therapy was given along with ART. She was advised calcium, vitamin D3, and folic acid supplementation. The patient was compliant in taking ART with >95% adherence to therapy and was under regular follow-up at the ART center at our tertiary care center. She had virological suppression with no viral load detected at the last testing on May 7, 2021, and a CD4 + count of 146/µL. Before this presentation, she had a history of fall at home 1.5 years back following which she had pain near the right hip region. She did not seek any medical advice at the time and the pain gradually resolved over the next 2–3 months. Apparently, she was not compliant in taking calcium and vitamin 3 supplements for the past few years. Radiology of the pelvis at the time of presentation shows healed superior and inferior pubic ramus fracture on the right side and healed superior pubic ramus fracture left side (Fig. 1b). There was no history to suggest other comorbid illnesses (apart from HIV infection) or the use of systemic glucocorticoids in the past. She had regular menstrual cycles. On physical examination, there was tenderness present over the proximal left thigh. The movements of the left hip joint could not be performed due to the pain whereas the same side knee and ankle showed a normal range of movements (ROMs). The right hip, knee, and ankle showed a normal ROM. The distal neurovascular status of both lower limbs was normal. Radiographs showed a left-sided transverse subtrochanteric (ST) closed femur fracture and an old right superior and inferior pubic rami fracture (Fig. 1). Skeletal traction was given with proximal tibial Steinman’s pin to stabilize the fracture. On presentation, the laboratory findings showed severe anemia (hemoglobin-6g/dl), hypophosphatemia (1.58 mg/dL), mild hypocalcaemia (8.41 mg/dL), vitamin D3 deficiency (5.8 ng/mL), normal PTH level (63.7 pg/mL), and an elevated alkaline phosphatase level (239 U/L). Her renal function parameters such as serum urea (19.8 mg/dL) and creatinine (0.71 mg/dL) were normal. Serum electrolyte levels such as sodium (135.2 mmoL/L), potassium (4.3 mmoL/L), and chloride (106.5 mmoL) were normal as well. Dual-energy X-ray absorptiometry (DEXA) scan showed normal BMD at the lumbar spine (T score of + 1.9) and right hip (T score of −0.1).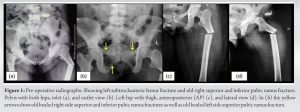
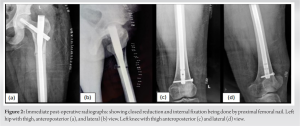
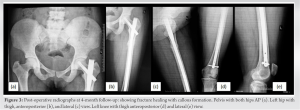
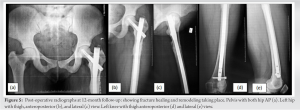
HIV infection itself has an adverse effect on bone health. The chronic inflammatory process associated with it causes the release of cytokines such as tumor necrosis factor-alpha, interleukin- 6, and the receptor activator of nuclear factor kappa-B ligand by the activated T- and B-cells. These cytokines lead to osteoclastogenesis resulting in bone resorption [4]. Apart from the proinflammatory state, traditional risk factors for abnormal bone health like alcohol abuse, nicotine consumption, opiate abuse, low BMI, and poor nutritional status are also commonly present in these patients [5]. Therefore, it is challenging to understand the role of HIV infection itself in causing skeletal fragility. To complicate things further, some drugs used as part of HAART can also affect bone health in patients with HIV infection [6]. Tenofovir disoproxil fumarate (TDF) is one of the first-line agents in HAART, and its long-term use has been shown to affect bone health in different ways. TDF, when present in high concentrations intracellularly, can lead to mitochondrial DNA toxicity within the proximal tubules of the nephron leading to renal dysfunction [7]. This proximal tubular dysfunction leads to urinary excretion of phosphorous and inhibition of 1a-hydroxylase which inhibits the synthesis of 1,25 (OH)2 vitamin D (calcitriol) [8]. The resultant hypophosphatemia, hypocalcaemia, and hypovitaminosis D cause the demineralization of bones, leading to osteoporosis. In our case, the renal function of the patient is preserved, and therefore, it is not a case of classical Fanconi syndrome; rather, it is a case of hypovitaminosis D-related osteomalacia. Table 1 describes the literature review of case reports previously published with tenofovir-induced osteomalacia and fragility fractures. The median age of the patients was 48 years (range 37–69 years). About 41% of the population was female. The median time from TDF start to the presentation of symptoms of the patients was 4 years (Range: 1 month–10 years). The majority of the studies (88%) have the clinical form of both osteomalacia and proximal renal tubulopathy. Tenofovir was withdrawn in all the studies. The median time to clinical improvement after tenofovir withdrawal was 3 months (range: 1–12 months) Hypophosphatemia was present in all the cases, 25% of patients (3/12) had hypocalcaemia, and 21.4% of patients (3/14) had hypovitaminosis. Borges et al. [9] noted that TDF itself was an independent risk factor for fracture. According to Rabenda et al. [10], there was no evident relationship between BMD changes and reduction in risk of fractures among patients receiving calcium with or without vitamin D supplementation. Calcium and/or vitamin D have an independent mechanism to reduce fracture rates which are not related to bone density [10]. Das et al. [11] found in their study that patients suffering from severe vitamin D deficiency were associated with raised PTH but no negative association with BMD was seen. But also, not all patients had raised PTH levels. To say patients with severe vitamin D deficiency with raised PTH were not always associated with abnormal BMD [11]. Current guidelines recommend the use of fracture risk assessment tool to assess fracture probability in PLHIV age ≥40 years and the measurement of bone mineral density in those at increased fracture risk. Vitamin D deficiency, if present, should be treated [12]. Ansemant et al. [13] pointed out that vitamin D supplementation may be beneficial in severe hypovitaminosis D in HIV patients. Some studies have shown that bisphosphonates can help in preventing bone loss due to ART [14] or treating osteoporosis in HIV-positive patients. However, clear guidelines for their use are still not available. The surgical treatment of PLHA patients is similar to the standard treatment of fragility fractures; additionally, there is no evidence of a significant difference in complication rates such as infection or consolidation [15]. In our case, we changed the HAART regimen where tenofovir was stopped. We also focused on monitoring the bone health and renal function of the patient and provided the patient with regular vitamin D supplements and compliance was ensured. We found over the next 12 months that the patient’s bone health markedly improved, and the patient achieved complete fracture union as well as functional status.
An HIV-positive patient under ART should be carefully monitored for drug-related complications such as renal dysfunction, osteomalacia, and osteoporosis. The prevention of fractures in these individuals is vital. The patient should receive care under a multidisciplinary approach to monitor bone quality, and find an appropriate HAART regimen. If any abnormality is detected, correct the metabolic disturbances, and provide a timely surgical treatment (in case of fracture) which will allow early movement and rehabilitation of the patient.
PLHA patients on a tenofovir-based HAART regimen need careful periodic monitoring for their bone health and renal function. Once there is any abnormality detected, the tenofovir-based HAART regimen needs to be changed along with osteomalacia treatment to prevent any pathological or fragility fractures.
References
- 1.Yang HY, Beymer MR, Suen SC. Chronic disease onset among people living with HIV and AIDS in a large private insurance claims dataset. Sci Rep 2019;9:18514. [Google Scholar]
- 2.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab 2008;93:3499-504. [Google Scholar]
- 3.Han WM, Wattanachanya L, Apornpong T, Jantrapakde J, Avihingsanon A, Kerr SJ, et al. Bone mineral density changes among people living with HIV who have started with TDF-containing regimen: A five-year prospective study. PLoS One 2020;15:e0230368. [Google Scholar]
- 4.Vikulina T, Fan X, Yamaguchi M, Roser-Page S, Zayzafoon M, Guidot DM, et al. Alterations in the immuno-skeletal interface drive bone destruction in HIV-1 transgenic rats. Proc Natl Acad Sci U S A 2010;107:13848-53. [Google Scholar]
- 5.Jones S, Restrepo D, Kasowitz A, Korenstein D, Wallenstein S, Schneider A, et al. Risk factors for decreased bone density and effects of HIV on bone in the elderly. Osteoporos Int 2008;19:913-8. [Google Scholar]
- 6.Mazziotti G, Canalis E, Giustina A. Drug-induced osteoporosis: Mechanisms and clinical implications. Am J Med 2010;123:877-84. [Google Scholar]
- 7.Lebrecht D, Venhoff AC, Kirschner J, Wiech T, Venhoff N, Walker UA. Mitochondrial tubulopathy in tenofovir disoproxil fumarate-treated rats. J Acquir Immune Defic Syndr 2009;51:258-63. [Google Scholar]
- 8.Van Rompay KK, Brignolo LL, Meyer DJ, Jerome C, Tarara R, Spinner A, et al. Biological effects of short-term or prolonged administration of 9-[2-(phosphonomethoxy)propyl]adenine (tenofovir) to newborn and infant rhesus macaques. Antimicrob Agents Chemother 2004;48:1469-87. [Google Scholar]
- 9.Borges ÁH, Hoy J, Florence E, Sedlacek D, Stellbrink HJ, Uzdaviniene V, et al. Antiretrovirals, fractures, and osteonecrosis in a large international HIV cohort. Clin Infect Dis 2017;64:1413-21. Erratum in: Clin Infect Dis 2017;65:1431-3. [Google Scholar]
- 10.Rabenda V, Bruyère O, Reginster JY. Relationship between bone mineral density changes and risk of fractures among patients receiving calcium with or without Vitamin D supplementation: A meta-regression. Osteoporos Int 2011;22:893-901. [Google Scholar]
- 11.Das S, Bopitya S, Taha H, David L. Relationship between Vitamin D, parathyroid hormone, bone mineral density, fracture and antiretroviral therapy in HIV patients. Recent Pat Antiinfect Drug Discov 2014;9:6-13. [Google Scholar]
- 12.Premaor MO, Compston JE. People living with HIV and fracture risk. Osteoporos Int 2020;31:1633-44. [Google Scholar]
- 13.Ansemant T, Mahy S, Piroth C, Ornetti P, Ewing S, Guilland JC, et al. Severe hypovitaminosis D correlates with increased inflammatory markers in HIV infected patients. BMC Infect Dis 2013;13:7. [Google Scholar]
- 14.Carr A, Kerr SJ, Richardson R, Ebeling P, Pocock N, Rojas J, et al. Prolonged effect of zoledronic acid on bone mineral density and turnover in HIV-infected adults on tenofovir: A randomized, open-label study. J Bone Miner Res 2019;34:2192-7. [Google Scholar]
- 15.Wijesekera MP, Graham SM, Lalloo DG, Simpson H, Harrison WJ. Fracture management in HIV positive individuals: A systematic review. Int Orthop 2016;40:2429-45. [Google Scholar]
- 16.Earle KE, Seneviratne T, Shaker J, Shoback D. Fanconi’s syndrome in HIV+adults: Report of three cases and literature review. J Bone Miner Res 2004;19:714-21. [Google Scholar]
- 17.Parsonage MJ, Wilkins EG, Snowden N, Issa BG, Savage MW. The development of hypophosphataemic osteomalacia with myopathy in two patients with HIV infection receiving tenofovir therapy. HIV Med 2005;6:341-6. [Google Scholar]
- 18.Perrot S, Aslangul E, Szwebel T, Caillat-Vigneron N, Le Jeunne C. Bone pain due to fractures revealing osteomalacia related to tenofovir-induced proximal renal tubular dysfunction in a human immunodeficiency virus-infected patient. J Clin Rheumatol 2009;15:72-4. [Google Scholar]
- 19.Di Biagio A, Rosso R, Monteforte P, Russo R, Rovetta G, Viscoli C. Whole body bone scintigraphy in tenofovir-related osteomalacia: A case report. J Med Case Rep 2009;3:8136. [Google Scholar]
- 20.Wanner DP, Tyndall A, Walker UA. Tenofovir-induced osteomalacia. Clin Exp Rheumatol 2009;27:1001-3. [Google Scholar]
- 21.Jhaveri MA, Mawad HW, Thornton AC, Mullen NW, Greenberg RN. Tenofovir-associated severe bone pain: I cannot walk! J Int Assoc Physicians AIDS Care (Chic) 2010;9:328-34. [Google Scholar]
- 22.Saidenberg-Kermanac’h N, Souabni L, Prendki V, Prie D, Boissier MC. Normal plasma FGF23 levels kinetic in tenofovir-related hypophosphatemic osteomalacia in an HIV-infected patient with von Recklinghausen disease. Joint Bone Spine 2011;78:306-8. [Google Scholar]
- 23.Haverkort ME, van der Spek BW, Lips P, Slieker WA, Heine RT, Huitema AD, et al. Tenofovir-induced Fanconi syndrome and osteomalacia in two HIV-infected patients: Role of intracellular tenofovir diphosphate levels and review of the literature. Scand J Infect Dis 2011;43:821-6. [Google Scholar]
- 24.De Socio GV, Fabbriciani G, Massarotti M, Messina S, Cecchini E, Marasini B. Hypophosphatemic osteomalacia associated with tenofovir: A multidisciplinary approach is required. Mediterr J Hematol Infect Dis 2012;4:e2012025. [Google Scholar]
- 25.Lovy M. AB0888 Tenofovir induced osteomalacia: A profile based on three patients with low phosphorus and normal levels of Vitamin D and parathyroid hormone. Ann Rheum Dis 2017;76:1366-7. [Google Scholar]
- 26.Kumar B, Prabhakar R, Thangavelu S. Tenofovir-induced delayed nephro-osteo toxicity. J R Coll Physicians Edinb 2020;50:291-4. [Google Scholar]