Biological therapy with adult live-cultured osteoblasts maybe a promising therapeutic option for AVN of the femoral head in patients with sickle cell anemia.
Dr. Alok Chandra Agrawal, Department of Orthopaedics, All India Institute of Medical Sciences, Raipur, Chhatisgarh, India. E-mail: dralokcagrawal@gmail.com
Introduction: The prevalence of avascular necrosis (AVN) of the femoral head in sickle cell anemia is 50% whereas untreated cases lead to total hip replacement. The recent development in cellular therapy paves the way to utilize autologous adult live-cultured osteoblasts (AALCO) in the management of AVN of the femoral head secondary to sickle cell anemia.
Case Report: We performed AALCO implantation in sickle cell anemia cases with AVN of the femoral head and were followed up for 6 months with the regular recording of visual analog score and modified Harris Hip Score.
Conclusion: AALCO implantation for the management of AVN of the femoral head due to sickle cell anemia appears to be the biological management of choice as it results in pain reduction and improvement in function.
Keywords: Osteonecrosis, femoral head, cultured osteoblasts, sickle cell anemia.
Avascular necrosis (AVN) of the femoral head in sickle cell disease (SCD) is the result of infarction caused due to occlusion of the microvasculature of the head. The deformed crescent-shaped RBCs in SCD are prone to adhere to endothelial and other cells, causing vaso-occlusion which progresses to bone marrow ischemia and AVN. The prevalence of AVN of the femur in patients with SCD is as high as 50% by the age of 35 years thus severely impacting their quality of life[1,2]. If left untreated, secondary arthritis of the hip is an inevitable consequence with the patients requiring total hip arthroplasty (THR). THR is associated with high rates of revision when performed at a younger age. Several strategies have been proposed to delay THR in SCD patients which include core decompression, platelet-rich plasma, bone marrow-derived mononuclear cells [3,4], and more recentlyautologous adult live-cultured osteoblast(AALCO) [5,6].This study aims to report the short-term clinical and radiological outcomes of AALCO in patients with SCD.
Participants
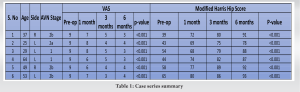
We present a series of six cases of osteonecrosis of the femoral head secondary to sickle cell anemia treated with AALCO implantation with a short-term follow-up of 6 months along with their functional and radiological outcome. Written informed consent was obtained from all patient’s parents before enrolment in the study. Six patients who were diagnosed to have sickle cell anemia with AVN of the femoral head according to modified Ficat and Arlet stage 0, 1, 2a, or 2b were included in this case series. Exclusion criteria included patients with AVN stage 3 and above, previous history of surgical intervention, history of chronic steroid use, acute recurrent painful crisis, and patients on immunosuppressive therapy. All patients presented with severe hip pain with restricted ROM and difficulty in carrying out their activities of daily living. The mean age of patients was 42.83 years, with mean pre-operative VAS and mHHS of 9 and 50.5, respectively.
AALCO procedure
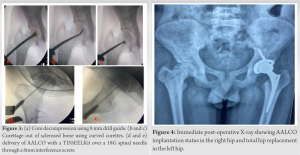
AALCO implantation is a two-stage procedure that firstly involves aspiration of about 12 mL of bone marrow from the right anterior iliac crest and transported to the Regrow Biosciences laboratory for ex-vivo MSC growth where MSCs are then redirected towards the osteoblastic lineage until the third passage. 48 million AALCO were collected in total per case. OSSGROW® (Regrow Biosciences Pvt Ltd., Mumbai, India) is a commercially FDA-approved technique that consists of implanting AALCOproduced from bone marrow aspirate mesenchymal stem cells for the treatment of AVN of the hip. The final product containing a highly characterized homogenous cell population is received from the laboratory after 3–4 weeks and is then injected into the patient’s hip after performing a core decompression and debridement of the affected hip using a TISSEELKit (Baxter, U.S.) under C-arm guidance.
Clinical and radiological evaluation
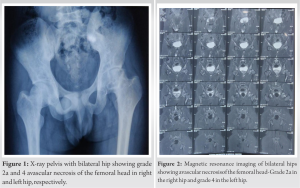
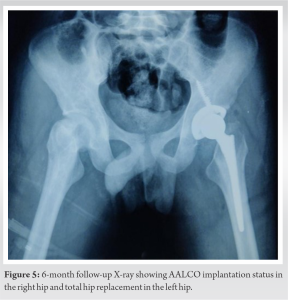
The patients were followed up at serial intervals at 2 weeks, 6 weeks, and 6 months. All patients were given the same rehabilitation, mobilization using walker support from day 2, partial weight bearing (50–80%), hip ROM, abduction exercises as tolerated till 6 weeks, and then full weight bearing walking with the return to normal activities. Patients reported a gradual reduction of pain and symptoms following the procedure and there was a gradual improvement in the mean modified Harris Hip Score (mHHS) and VAS which was found to be statistically significant (P<0.05) (Table 1). All patients reported significant improvement in their quality of life and were able to carry out all their routine daily activities without much discomfort or assistance. Radiographs at serial follow-up after AALCO implantation did not show any evolution of the lesion size or stage; however, this maybe attributed to the short duration of follow-up. A representational case image of the outcome of AALCO in SCD patients is shown in Fig. 1, 2, 3, 4, 5.
This case series showcases six cases of AVN of the femoral head due to SCD managed using AALCO implantation. The implantation procedure showed good outcomes both in terms of clinical improvement as well as delaying radiological progression in patients AVN of the hip due to SCD upto Ficat–Arlet stage 2B. AVN is a rapidly advancing disease, and the conservative treatment of bed rest and restricted weight bearing has been shown to have only a 20% success rate in halting disease progression. AALCO implantation has previously shown good treatment outcomes in patients with idiopathic AVN and steroid-induced AVN [7,8]; however, not much evidence exists regarding its efficacy in the management of AVN due to SCD. Hernigou et al. in their study on the natural progression of AVN in sickle cell patients showed that the mean time from diagnosis to the collapse of the femoral head in SCD was 42 months and 30 months in stage1 and stage 2, respectively, which highlights the need for early intervention to delay disease progression [9]. The benefits of autologous bone marrow concentrate injections have been demonstrated in multiple studies showing significant improvement in pain, clinical outcome, and reducing the progression of the disease for as long as 17 years [5,10]. The beneficial effects of this cell-based therapy have been attributed to the delivery of progenitor cells and stem cells to enrich the local micro-environment by promoting angiogenesis and osteogenesis [11]. The outcome of the procedure has also shown to be dependent on the activity and quantity of the MSCs delivered. Patients with steroid and alcohol abuse are shown to have a reduced number of granulocyte-macrophage progenitor cells as well as fibroblast colony-forming units in their bone marrow [12,13].However, in the early stages of AVN in SCD, it is found that cell differentiation abilities, replication, and production of growth factors and cytokines are not altered and the ex-vivo expansion of MSCs did not alter their replicative capacity or biomolecular characteristics, making AALCO one of the most ideal cell-based therapies to promote bone repair and healing in the early stages of AVN [5]. Furthermore, the risk of allogeneic stem cells causing tumors and heterotopic ossification can be eliminated with the use of autologous cell therapy which has been proven in studies with long-term follow-ups [14]. Our results agreed with Sadat-Ali et al., who showed significant improvement both in VAS and mHHS at serial follow-ups in SCD patients treated with AALCO [15].The main limitations of this study include its small size and short duration of follow-up. Further large-scale prospective studies with comparative groups involving core decompression alone or conservative management with long-term follow-ups may offer better insights into the efficacy of this treatment modality.
AALCO, which uses a minimally invasive technique to deliver the patients’ cultured osteoblasts, may offer a viable therapeutic strategy in patients suffering from AVN of the femoral head due to SCD, potentially postponing disease progression and the necessity for total hip replacement.
In 6-month follow-up, AALCOs offer better pain relief and functional outcomes in patients with avascular necrosis of the femoral head due to sickle cell anemia.
References
- 1.Mahadeo KM, Oyeku S, Taragin B, Rajpathak SN, Moody K, Santizo R, et al. Increased prevalence of osteonecrosis of the femoral head in children and adolescents with sickle-cell disease. Am J Hematol 2011;86:806-8. [Google Scholar]
- 2.Hernigou P, Habibi A, Bachir D, Galacteros F. The natural history of asymptomatic osteonecrosis of the femoral head in adults with sickle cell disease. J Bone Joint Surg Am 2006;88:2565-72. [Google Scholar]
- 3.Ma Y, Wang T, Liao J, Gu H, Lin X, Jiang Q, et al. Efficacy of autologous bone marrow buffy coat grafting combined with core decompression in patients with avascular necrosis of femoral head: A prospective, double-blinded, randomized, controlled study. Stem Cell Res Ther 2014;5:115. [Google Scholar]
- 4.Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: Five year follow-up of a prospective controlled study. Bone 2011;49:1005-9. [Google Scholar]
- 5.Daltro GC, Fortuna V, De Souza ES, Salles MM, Carreira AC, Meyer R, et al. Efficacy of autologous stem cell-based therapy for osteonecrosis of the femoral head in sickle cell disease: A five-year follow-up study. Stem Cell Res Ther 2015;6:110. [Google Scholar]
- 6.Li X, Xu X, Wu W. Comparison of bone marrow mesenchymal stem cells and core decompression in treatment of osteonecrosis of the femoral head: A meta-analysis. Int J Clin Exp Pathol 2014;7:5024-30. [Google Scholar]
- 7.Palekar G, Bhalodiya HP, Archik S, Trivedi K. Retrospective study on implantation of autologous-cultured osteoblasts for the treatment of patients with avascular necrosis of the femoral head. Orthop Res Rev 2021;13:15-23. [Google Scholar]
- 8.Hernigou P, Beaujean F, Lambotte JC. Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg Br 1999;81:349-55. [Google Scholar]
- 9.Hernigou P, Bachir D, Galacteros F. The natural history of symptomatic osteonecrosis in adults with sickle-cell disease. J Bone Joint Surg Am 2003;85:500-4. [Google Scholar]
- 10.Hernigou P, Daltro G, Filippini P, Mukasa MM, Manicom O. Percutaneous implantation of autologous bone marrow osteoprogenitor cells as treatment of bone avascular necrosis related to sickle cell disease. Open Orthop J 2008;2:62-5. [Google Scholar]
- 11.Yan Z, Hang D, Guo C, Chen Z. Fate of mesenchymal stem cells transplanted to osteonecrosis of femoral head. J Orthop Res 2009;27:442-6. [Google Scholar]
- 12.Hernigou P, Beaujean F. Abnormalities in the bone marrow of the iliac crest in patients who have osteonecrosis secondary to corticosteroid therapy or alcohol abuse. J Bone Joint Surg Am 1997;79:1047-53. [Google Scholar]
- 13.Lee JS, Lee JS, Roh HL, Kim CH, Jung JS, Suh KT. Alterations in the differentiation ability of mesenchymal stem cells in patients with nontraumatic osteonecrosis of the femoral head: Comparative analysis according to the risk factor. J Orthop Res 2006;24:604-9. [Google Scholar]
- 14.Wakitani S, Okabe T, Horibe S, Mitsuoka T, Saito M, Koyama T, et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med 2011;5:146-50. [Google Scholar]
- 15.Sadat-Ali M, Al-Omran AS, AlTabash K, Acharya S, Hegazi TM, Al Muhaish MI. The clinical and radiological effectiveness of autologous bone marrow derived osteoblasts (ABMDO) in the management of avascular necrosis of femoral head (ANFH) in sickle cell disease (SCD). J Exp Orthop 2022;9:18. [Google Scholar]