Expansile lytic lesions in atypical locations should be taken seriously and the diagnosis can be easily missed if the clinician is not aware of the wide array of differential diagnosis which range from an infectious etiology like tuberulous osteomyelitis to a neoplastic etiology like giant cell tumor.
Dr. Pranav Gupta, Department of Orthopaedics, Guru Gobind Singh Medical College and Hospital, Faridkot - 151 203, Punjab, India. E-mail: pranavchd88@gmail.com
Introduction: Giant cell tumor (GCT) represents 5% of all primary bone tumors and 20% of biopsy analyzed benign tumors. More than half of these lesions occur in the 3rd and 4th decades of life. There is no absolute treatment method selection. Techniques ranging from intralesional curettage to wide resection can be used. Goal is to eradicate the tumor, preserve limb function, and prevent local recurrence and distant metastasis.
Case Report: We are presenting seven cases of GCT at five different and rare sites involving tibia, calcaneum, metatarsal, proximal humerus, and clavicle with tumor being limited to bone in all seven cases not involving the soft tissue. There were three male patients and four female patients. Six patients underwent intralesional curettage using high-speed burr and curette, along with adjuvant irrigation with hydrogen peroxide and normal saline followed by polymethyl methacrylate reconstruction. One patient with GCT clavicle underwent wide resection.
Results: In all seven cases, we were able to able to remove the tumor completely. Six patients had a gradual and complete recovery with return to near normal activity within 6 month–1 year after surgery. One patient with proximal humerus GCT had a recurrence which got resolved with injection denosumab. All patients have been followed up for a minimum duration of 2 years.
Conclusion: Intervention in the early stages can avoid radical procedures such as wide local excision or amputation. We recommend aggressive surgical approach with close follow-up to detect recurrence if any, at an early stage.
Keywords: Burr, cementing, curettage, denosumab, giant cell tumor.
Giant cell tumor (GCT) represents approximately 5% of all primary bone tumors and 20% of biopsy analyzed benign tumors [1, 2]. More than half of these lesions occur in the 3rd and 4th decades of life [1] These lesions commonly develop in patients between 20 and 40 years of age, with a slight female predominance [2]. About 85–90% of GCT usually occurs in the meta-epiphyseal region of long bones out of which 50% occurs around the knee followed by distal radius, sacrum, axial skeleton, and small bones of the hands and feet [2, 3, 4]. Despite being nonfatal, benign bone tumors may lead to considerable disruption of the local bony architecture that can be particularly harmful around joints. Bone disruption can be particularly problematic around joints, compromising joint function and mobility [5]. There is no absolute treatment method selection. Techniques ranging from intralesional curettage to wide resection can be used. Goal is to eradicate the tumor, preserve limb function, and prevent local recurrence and distant metastasis. Although nearly 100% local control is attained with en bloc resection, this procedure is often associated with functional loss due to the periarticular location of many of GCTs [6, 7]. Hence, intralesional curettage is considered standard treatment. However, GCT has a high possibility to recur after curettage with rates between 25% and 50% [2]. Hence, many adjuvant treatments have been applied to decrease the recurrence rates [2]. These include liquid nitrogen, acrylic cement polymethyl methacrylate (PMMA), phenol, and hydrogen peroxide [2]. Sparing of joint and subchondral bone makes GCT lesion a very good contender for adjuvant bone cement reconstruction after an intralesional curettage with bone defects attaining a mechanical strength to 98% and recurrence rate of about 22% [8]. Cytotoxic and thermal effects of bone cement on remaining microscopic disease and easier radiographic detection of recurrence are other advantages of bone cement reconstruction [9]. We are presenting our experience with six patients, of which two each were diagnosed with GCT of calcaneum and proximal humerus, respectively, and one each with GCT of 1st metatarsal and distal tibia, respectively, all of which are very rare site for such tumors.
We are presenting seven cases of GCT with one patient involving distal tibia, two patients involving calcaneum. One involving 1st metatarsal and two patients involving proximal humerus and one patient involving clavicle with tumor being limited to bone in all six cases not involving the soft tissue (Table 1).
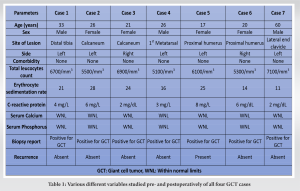
All patients were operated at Guru Gobind Singh Medical College and Hospital, a tertiary referral hospital located in Faridkot, Punjab between 2019 and 2020 and were followed up for a period of 2 years postoperatively. There were three male patients and four female patients. All seven patients presented with swelling in the involved region of long duration which was insidious in onset and had gradually increased in size with dull aching pain mild to moderate in intensity which was relieved by taking medication. There was no history of any constitutional symptoms and trauma in any of the patient. In all six cases, swelling was tender, firm in consistency, and the overlying skin was not tethered. Preoperatively, X-ray and magnetic resonance imaging (MRI) were done in which expansile lytic lesion was seen in the respective bone; however, subchondral bone was intact and lesion had not infiltrated the surrounding soft tissue. Chest X-ray did not reveal any metastatic deposit in any patient. The following variables were extracted from the medical records: Age at the time of admission, sex, symptoms at presentation, comorbidities, surgical details, history of trauma, bacteriology, and relevant medical history. In chronological order, first patient had lesion in distal tibia, second and third patient had lesion in calcaneum, fourth patient had lesion involving first metatarsal, fifth and sixth patient had lesion involving proximal humerus, and seventh patient had lesion involving lateral end of clavicle. There were no associated comorbidities in any patient. The following laboratory values at presentation were collected: Complete blood count (cells/mm3), erythrocyte sedimentation rate (mm/h), and C-reactive protein (mg/L) to rule out any infective ethology along with serum calcium and serum phosphorus which were within normal limits. Needle biopsy under image guidance was done and samples were sent for histopathology which confirmed GCT. Following this surgery was planned. Six patients underwent intralesional curettage using high-speed burr and curette along with adjuvant irrigation with hydrogen peroxide and normal saline followed by PMMA cement reconstruction, while in seventh patient with GCT clavicle, wide excision was done. In one 17-year-old patient with GCT proximal humerus, we also used denosumab injection as neoadjuvant therapy and also postoperatively for recurrence. In first case, a 33-year-old male patient presented with complain of pain and swelling on the distal aspect of the left leg for the past 1 year associated with severe pain which was aggravated on walking. History of trauma, fever, loss of weight, or any other constitutional symptom were absent. On examination, bony hard swelling was present over lateral aspect of the left distal leg about 5 × 4 cm in size with well define margins, with smooth surface and normal overlying skin. Swelling was tender on palpation with no local rise of temperature. Movement around ankle was normal. Plane radiograph and MRI of the left ankle showed subarticular expansile lytic lesion at distal end of the left tibia suggestive of GCT (Fig. 1a and b). Intralesional curettage with bone cement reconstruction was done by giving an anterior incision over the distal end of the tibia and making a cortical window of 3 cm × 2 cm size (Fig. 1c-e). The tumor was sent for histopathological examination which confirmed the diagnosis of GCT. The patient was put on a below knee plaster of Paris cast after stitch removal and was removed after 3 months postoperatively, and partial weight-bearing was started which was increased gradually. X-rays were taken at regular follow-up. The patient has followed up regularly since last 2 years with no recurrence. In second case, a 26-year-old female presented to opd with complaint of pain and swelling in the left heel since 6 months. On examination, there was a well-defined bony hard swelling of 5 cm × 2 cm size over the posterior aspect of the left heel with normal overlying skin. On presentation, a radiograph was taken which showed 5 cm × 3 cm × 3 cm size osteolytic expansile lesion in the left calcaneus with intact cortical margins (Fig. 2a). The patient underwent curettage with burring and hydrogen peroxide along with cementing and curetted material was sent for biopsy. The patient was put on a below knee plaster of Paris cast for 6 weeks. Gradual weight-bearing was started 6 weeks onward on a weight-bearing cast. The patient was followed up every month for first 6 months and every 3 months for next 18 months. In this 2-year follow-up, the patient remained disease free and ambulatory. No recurrence of symptoms or no new growth was observed (Fig. 2b).
On examination, bony hard swelling was present over lateral aspect of the left distal leg about 5 × 4 cm in size with well define margins, with smooth surface and normal overlying skin. Swelling was tender on palpation with no local rise of temperature. Movement around ankle was normal. Plane radiograph and MRI of the left ankle showed subarticular expansile lytic lesion at distal end of the left tibia suggestive of GCT (Fig. 1a and b). Intralesional curettage with bone cement reconstruction was done by giving an anterior incision over the distal end of the tibia and making a cortical window of 3 cm × 2 cm size (Fig. 1c-e). The tumor was sent for histopathological examination which confirmed the diagnosis of GCT. The patient was put on a below knee plaster of Paris cast after stitch removal and was removed after 3 months postoperatively, and partial weight-bearing was started which was increased gradually. X-rays were taken at regular follow-up. The patient has followed up regularly since last 2 years with no recurrence. In second case, a 26-year-old female presented to opd with complaint of pain and swelling in the left heel since 6 months. On examination, there was a well-defined bony hard swelling of 5 cm × 2 cm size over the posterior aspect of the left heel with normal overlying skin. On presentation, a radiograph was taken which showed 5 cm × 3 cm × 3 cm size osteolytic expansile lesion in the left calcaneus with intact cortical margins (Fig. 2a). The patient underwent curettage with burring and hydrogen peroxide along with cementing and curetted material was sent for biopsy. The patient was put on a below knee plaster of Paris cast for 6 weeks. Gradual weight-bearing was started 6 weeks onward on a weight-bearing cast. The patient was followed up every month for first 6 months and every 3 months for next 18 months. In this 2-year follow-up, the patient remained disease free and ambulatory. No recurrence of symptoms or no new growth was observed (Fig. 2b).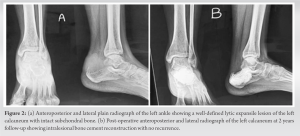 The patient is able to do full weight-bearing (FWB) walking with support of stick. Third case was of a 21-year-old female who presented to opd with complaint of pain and swelling in the right heel since past 8 months. On examination, there was a well-defined bony hard swelling of 4 × 3 cm size over the right heel with normal overlying skin. The patient had no past history of trauma or fall. On presentation, a radiograph was taken which showed 4 cm × 2 cm × 2 cm size osteolytic expansile lesion in the right calcaneus with intact cortical margin. Post curettage and cementing patient was put on a below knee plaster of Paris cast for 6 weeks. Gradual weight-bearing was started 6 weeks onward on a weight-bearing cast. The patient was followed up every month for first 6 months, followed by every 3 months from next 18 months. In this 2-year follow-up, the patient remained disease free and ambulatory, no recurrence of symptoms or no new growth was observed. At present, the patient is able to do FWB walking with support of stick. Fourth case was of a 26-year-old male who presented to us with swelling on dorsomedial aspect of the left foot since 12 months and pain for the past 6 months. Earlier patient was taking treatment from quack who gave him pain killers and antibiotics. However, there was no improvement and swelling and pain continued to increase. There is no history of any constitutional symptoms or trauma. On examination, there was a well-defined bony hard swelling of 3 × 2 cm size over the dorsomedial aspect of the left foot opposing 1st metatarsal with well-defined margins, stretched overlying skin and without any sign of inflammation. Radiograph was taken which showed 3 cm × 2 cm × 1 cm size osteolytic lesion in the first metatarsal with intact cortical margins (Fig. 3a). Post-operative X-ray was satisfactory. The patient was given a below knee cast for 3 months postoperatively.
The patient is able to do full weight-bearing (FWB) walking with support of stick. Third case was of a 21-year-old female who presented to opd with complaint of pain and swelling in the right heel since past 8 months. On examination, there was a well-defined bony hard swelling of 4 × 3 cm size over the right heel with normal overlying skin. The patient had no past history of trauma or fall. On presentation, a radiograph was taken which showed 4 cm × 2 cm × 2 cm size osteolytic expansile lesion in the right calcaneus with intact cortical margin. Post curettage and cementing patient was put on a below knee plaster of Paris cast for 6 weeks. Gradual weight-bearing was started 6 weeks onward on a weight-bearing cast. The patient was followed up every month for first 6 months, followed by every 3 months from next 18 months. In this 2-year follow-up, the patient remained disease free and ambulatory, no recurrence of symptoms or no new growth was observed. At present, the patient is able to do FWB walking with support of stick. Fourth case was of a 26-year-old male who presented to us with swelling on dorsomedial aspect of the left foot since 12 months and pain for the past 6 months. Earlier patient was taking treatment from quack who gave him pain killers and antibiotics. However, there was no improvement and swelling and pain continued to increase. There is no history of any constitutional symptoms or trauma. On examination, there was a well-defined bony hard swelling of 3 × 2 cm size over the dorsomedial aspect of the left foot opposing 1st metatarsal with well-defined margins, stretched overlying skin and without any sign of inflammation. Radiograph was taken which showed 3 cm × 2 cm × 1 cm size osteolytic lesion in the first metatarsal with intact cortical margins (Fig. 3a). Post-operative X-ray was satisfactory. The patient was given a below knee cast for 3 months postoperatively.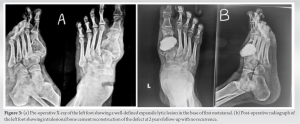 FWB was started after 3 months. Thereafter, the patient was followed up after every 3 months with check X-ray which did not show any recurrence. The patient is on our regular follow-up since last 2 years and is able to walk without pain with the support of stick (Fig. 3b). The patient has returned to normal activities without much limitation. Fifth case was of a 17-year-old female who presented to us with complaint of pain and swelling of the left shoulder for 2 years. On examination, there was bony hard swelling of size 5 × 5 cm over the left shoulder with minimal restriction of movement and normal and free overlying skin. On presentation, a radiograph was taken which showed 5 cm × 4 cm × 3 cm size osteolytic lesion in the left proximal humerus with intact cortical margins (Fig. 4a).
FWB was started after 3 months. Thereafter, the patient was followed up after every 3 months with check X-ray which did not show any recurrence. The patient is on our regular follow-up since last 2 years and is able to walk without pain with the support of stick (Fig. 3b). The patient has returned to normal activities without much limitation. Fifth case was of a 17-year-old female who presented to us with complaint of pain and swelling of the left shoulder for 2 years. On examination, there was bony hard swelling of size 5 × 5 cm over the left shoulder with minimal restriction of movement and normal and free overlying skin. On presentation, a radiograph was taken which showed 5 cm × 4 cm × 3 cm size osteolytic lesion in the left proximal humerus with intact cortical margins (Fig. 4a). MRI showed a T2 hyperintense expansile mass. The humeral head was deformed, but the lesion was restricted to bone. There was no soft-tissue involvement. Needle biopsy confirmed GCT. The patient also requested to try denosumab as a neoadjuvant treatment after we presented to him a few recent reports which showed that denosumab could induce ossification in GCT. Informed consent was obtained, and the patient received three doses of 120 mg each of denosumab subcutaneous injection in December 2018, February 2019, and April 2019. In July 2019, the patient underwent intralesional curettage +cementing. Post-operative X-ray showed no residual lytic lesion. However, 6 months later, the patient developed local recurrence (Fig. 4b). The patient was again given three doses of denosumab injection as neoadjuvant treatment following which we were planning curettage and cementing again, however, when the patient was followed up with serial X-rays at 2 months after the last injection, lesion was found to be resolved. The patient was then followed at 6 months interval for next 1½ year followed by MRI. In the literature, the use of injection denosumab for recurrence has not been reported. Fortunately, in our case, results were excellent with total remission (Fig. 4c). She was put on calcium and multivitamin supplement, and regular blood tests showed normal renal function and calcium level. The patient did not experience any side effect. Postoperatively, the patient has had a pain-free limb and is able to actively perform movements at the shoulder without any difficulty. Shoulder could flex up to 100°, extend up to 40°, and abduct up to 100°. Sixth case was of a 20-year-old female presenting to us with complaint of pain and swelling of the right shoulder since 2 years. On examination, there was bony hard swelling of size 6 × 4 cm over the right shoulder with minimal restriction of range of motion and normal and free overlying skin. On presentation, a radiograph was taken which showed 6 cm × 4 cm × 4 cm size osteolytic lesion in the right proximal humerus with intact cortical margins which were also seen on MRI. However, the lesion remained intraosseous. Needle biopsy of the lesion confirmed GCT. The patient underwent intralesional curettage +cementing. Post-operative X-ray showed no residual lytic lesion. The patient was followed up every 3 months. At 2 years postoperatively, the patient is pain free with ability to perform daily activities without any difficulty with no recurrence. Seventh case was of a 60-year-old male presenting to our department with pain and swelling over lateral end of the left clavicle. The selling was gradually increasing in size since past 4 months. On palpation, the swelling was tender, lobulated, and hard in consistency. The overlying skin was non-adherent and freely mobile. There was no regional lymphadenopathy and no neurovascular deficit in the left upper limb. We got a plain radiograph which revealed an expansile radiolucent lesion arising from lateral end of the left clavicle (Fig. 5).
MRI showed a T2 hyperintense expansile mass. The humeral head was deformed, but the lesion was restricted to bone. There was no soft-tissue involvement. Needle biopsy confirmed GCT. The patient also requested to try denosumab as a neoadjuvant treatment after we presented to him a few recent reports which showed that denosumab could induce ossification in GCT. Informed consent was obtained, and the patient received three doses of 120 mg each of denosumab subcutaneous injection in December 2018, February 2019, and April 2019. In July 2019, the patient underwent intralesional curettage +cementing. Post-operative X-ray showed no residual lytic lesion. However, 6 months later, the patient developed local recurrence (Fig. 4b). The patient was again given three doses of denosumab injection as neoadjuvant treatment following which we were planning curettage and cementing again, however, when the patient was followed up with serial X-rays at 2 months after the last injection, lesion was found to be resolved. The patient was then followed at 6 months interval for next 1½ year followed by MRI. In the literature, the use of injection denosumab for recurrence has not been reported. Fortunately, in our case, results were excellent with total remission (Fig. 4c). She was put on calcium and multivitamin supplement, and regular blood tests showed normal renal function and calcium level. The patient did not experience any side effect. Postoperatively, the patient has had a pain-free limb and is able to actively perform movements at the shoulder without any difficulty. Shoulder could flex up to 100°, extend up to 40°, and abduct up to 100°. Sixth case was of a 20-year-old female presenting to us with complaint of pain and swelling of the right shoulder since 2 years. On examination, there was bony hard swelling of size 6 × 4 cm over the right shoulder with minimal restriction of range of motion and normal and free overlying skin. On presentation, a radiograph was taken which showed 6 cm × 4 cm × 4 cm size osteolytic lesion in the right proximal humerus with intact cortical margins which were also seen on MRI. However, the lesion remained intraosseous. Needle biopsy of the lesion confirmed GCT. The patient underwent intralesional curettage +cementing. Post-operative X-ray showed no residual lytic lesion. The patient was followed up every 3 months. At 2 years postoperatively, the patient is pain free with ability to perform daily activities without any difficulty with no recurrence. Seventh case was of a 60-year-old male presenting to our department with pain and swelling over lateral end of the left clavicle. The selling was gradually increasing in size since past 4 months. On palpation, the swelling was tender, lobulated, and hard in consistency. The overlying skin was non-adherent and freely mobile. There was no regional lymphadenopathy and no neurovascular deficit in the left upper limb. We got a plain radiograph which revealed an expansile radiolucent lesion arising from lateral end of the left clavicle (Fig. 5). 
The swelling demonstrated geographic type destruction without any soft-tissue component or periosteal reaction. MRI was obtained which also suggested giant cell tumor. To aid in the diagnosis, fine needle aspiration cytology was done which confirmed giant cell tumor. Since the clavicle does not necessary require reconstruction and the patient was a retired school teacher, not engaged in any physical work, so surgical resection of the tumor was planned. A wide excision of the mass along with 3 cm of the healthy tissue was done. The excised mass was sent for histopathological examination which also confirmed it to be a GCT. No radiotherapy or chemotherapy was given postoperatively. Wound healing was uneventful. The range of motion of the left shoulder was normal, and postoperatively, there was no neurovascular deficit. The patient was happy with the surgical outcome and at 2 year follow-up that there was no evidence of recurrence or metastasis.
In six cases, we were able to able to remove the tumor completely without compromising the joint as subchondral bone was intact in all those cases. In the seventh case, tumor was excised en bloc all seven patients that had a gradual and complete recovery with return to near normal activity within 1 to 1½ years after surgery. All patients have been followed up for a minimum duration of 2 years and no recurrence has been seen in this period except in one patient. One patient with proximal humerus GCT in which recurrence was seen was give three doses of injection denosumab at 2 months interval as neoadjuvant treatment following which we were planing curettage and cementing, however lesion resolved within 6 months. Hence, the patient was followed up for a period of 2 years and has not shown any recurrence till date. We had no major complication in any case. Patients did have pain at the operative site postoperatively which gradually subsided. All seven cases showed gradual healing on subsequent follow-ups with improvement in activity level as the time passed and monitoring was done at fixed interval based on plain radiographs to look for any recurrence. Overall, patients are free of pain and made a good recovery in terms of mobility and joint function. Patients are still under regular follow-up.
GCT is generally seen in skeletally mature patients with maximum incidence in third decade with a male:female ratio of 1:1.5 [10]. In our case series, six out of seven cases were in the 21–30-year age group, one was 60 years old. Three were male and four were female. GCT is a rare, benign however locally aggressive bone tumor that may affect almost any bone, but is most common around the knee [2, 3, 4]. In our case series, we are reporting seven cases of bone GCT with one patient involving distal tibia, two patients involving calcaneum, one involving first metatarsal, two patients involving proximal humerus, and one patient involving lateral end of clavicle. Proximal humerus GCT accounts for only around 4% of the disease [11]. Furthermore, GCT in metatarsal bones is rare with incidence reported around 1.5% [4]. GCT around foot and ankle is also rare and comprises <4% of all GCT [12]. In a study performed by Campanacci et al., two cases out of the total of 327 were reported in the calcaneum, whereas Dahlin reported four out of 411 cases in his study; overall incidence being approximately 1% [13]. GCT of clavicle is rare with only 15 cases reported in literature in the past 40 years [14]. Errani et al. found no GCTs arising in the clavicula among 349 GCTs of bone [15]. Furthermore, national bone tumor registry in Japan reported only two cases of GCTs in the clavicula (1.1%) from 2006 to 2012 [16]. Treatment of GCT is directed toward local control without sacrificing joint function. This has traditionally been achieved by extended curettage which is currently the treatment of choice [2, 10]. Other treatment modalities include en bloc resection followed by reconstruction [17]. Due to the paucity of the available literature, no definite treatment guidelines are available on the management of GCT of clavicle. Partial or total claviculectomy seemed a reasonable option to us to treat GCT clavicle. Some authors have established that total or subtotal excision of clavicle was rarely associated with loss of function [18]. Usually, curettage is done for Campanacci grades I and II, while excision is carried out in Campanacci grade III [17]. First six cases included in this study were Campanacci grade 2 due to which curettage was performed in all six patients while seventh case was Campanacci grade III due to which excision was planned. Intralesional curettage usually leaves microscopic disease in the bone and, hence, has a recurrence rate of around 60% with most cases presenting within 2 years of primary surgery [15, 13, 19]. Hence, the addition of adjuvant treatments such as mechanical burr drilling of the tumor is recommended. Furthermore, the use of adjuvant like liquid nitrogen, acrylic cement, phenol, and hydrogen peroxide has lowered the rate of recurrence considerably [2]. We in first six cases used burr, hydrogen peroxide, and PMMA cement as adjuvants. PMMA reconstruction of the defect after intralesional curettage restores subchondral stiffness within 98% of the contralateral limb and, thus, provides immediate stability helping in rapid weight-bearing [2, 8]. Apart from this, PMMA cement due to its exothermic reaction and toxic effects on tumor cells helps to extend the boundary of tumor kill which helps in reducing recurrence rates [20]. Furthermore, radiographic detection of recurrence is easier. Some studies have shown a reduced recurrence rate after PMMA cementation [21, 22]. Scandinavian sarcoma group study also reported a lower local recurrence rate with PMMA cementation than without cementation [23]. Kivioja et al. and Becker et al. in their respective studies in 2008 also concluded that PMMA must be used to decrease recurrence rates in GCT of bone [23, 24]. One drawback of utilizing cement near the joint is the possibility of damage to articular cartilage which could lead to joint degeneration occurring at an early stage. Bini et al. found that 11% of patients suffered arthritis after cementing in subchondral area [25]. However, Wada et al. reported only one case where the patient developed osteoarthritis of the knee joint that too 14 years after surgery [26]. They highlighted that articular cartilage being intact preoperatively is more important which was there in all six patients reported in our study [15]. As a result, arthritis was not seen in any of our patients even at 2 years postoperatively. However, the local recurrence rate is still around 15–27% even with the use of an adjuvant [1]. Two recent studies have reported recurrence rates of 6%–17%, respectively [11, 27]. Another study has seen minimum one local recurrence in 14% of patients even with use of adjuvants [20]. One patient in our study with GCT proximal humerus also reported local recurrence after curettage and cementing. Recurrence rate is almost zero with en bloc resection as can be seen in patient with GCT clavicle. Recently, denosumab, a monoclonal antibody that targets receptor activator of nuclear factor k-B, has been used to treat GCT of bone. It has been shown to inhibit the osteoclastic activity of GCT, hence, is been used preoperatively to facilitate the recession of tumor as well as primary treatment for patients unwilling/unfit for surgery [28]. Around 65–80% of the tumor would show a significantly increased fibro-osseous tissue or new bone formation after denosumab treatment [29]. Mak et al. showed that a specimen from patients who had completed denosumab treatment still showed the presence of stromal cells concluding that that denosumab cannot be used as the sole treatment for GCT [30]. One patient in our study with proximal humerus GCT who underwent curettage and cementing developed recurrence as translucency was visible between bone and cement. Recurrence was most probably be due to incomplete removal of residual tumor during curettage. For this, we again gave three doses of injection denosumab as neoadjuvant at 1 month interval to the patient and were planning repeat curettage. However, when patient was followed up with serial X-rays at 2 months after the last injection, lesion was found to be resolved so we did not proceed with curettage and cementing and continued observing the patient every 3–6 months. Fortunately, there has been no recurrence since past 2 years. Our patient represented a good responder to denosumab treatment. It has been recommended that the use of bone graft under the cartilage may prevent the injury due to cement; but there are no statistically difference in functional outcome when either cement or bone graft is used adjacent to the cartilage after curettage [11]. However, bone grafting should be avoided in large and juxta-articular lesions as a lot of bone graft would be required to fill the defect which would lead to donor site morbidity. Furthermore, there are concerns regarding risk of disease transmission (allograft), and difficulty in visualizing (in X-rays) recurrence with grafts occupying the cavity. Furthermore, bone grafting alone will not give subchondral strength instantaneously which may cause chondral fracture or failure [20]. As a result, bone grafting was not done in any of our patients. However, there are certain limitations to our study. Sample size taken in this study is very small to reach a proper conclusion. Furthermore, in this study, patients were followed up for a duration of 2 years which is not enough to accurately comment on the recurrence in GCT cases.
GCT occurring in the above mentioned sites is a very unusual. It may present with pain or remain asymptomatic and come across by chance on a radiograph. Therefore, expansile lytic lesions in these atypical locations should be taken seriously and the diagnosis can be easily missed if the clinician is not aware of the wide array of differential diagnoses which range from an infectious etiology like tuberculous osteomyelitis to a neoplastic etiology like giant cell tumor. The typical appearance makes it easy to be diagnosed on simple radiographs. Denosumab has the potential to eliminate osteolysis and can be used to treat recurrence. The drug is usually well-tolerated. Intervention in early stages can avoid radical procedures such as wide excision and amputation. We recommend aggressive surgical approach with close follow-up to detect recurrence if any, at an early stage.
Expansile lytic lesions in these atypical locations should be taken seriously and the diagnosis can be easily missed if the clinician is not aware of the wide array of differential diagnosis.
References
- 1.Sobti A, Agrawal P, Agarwala S, Agarwal M. Giant cell tumor of bone-an overview. Arch Bone Jt Surg 2016;4:2-9. [Google Scholar]
- 2.Tejwani SG, Hame SL, Eckardt JJ. Subchondral giant-cell tumor of the proximal tibia: Arthroscopic treatment for accelerated articular cartilage and meniscal degeneration in two patients. Arthroscopy 2004;20:644-9. [Google Scholar]
- 3.van der Heijden L, Dijkstra PD, van de Sande MA, Kroep JR, Nout RA, van Rijswijk CS, et al. The clinical approach toward giant cell tumor of bone. Oncologist 2014;19:550-61. [Google Scholar]
- 4.Siddiqui YS, Zahid M, Bin Sabir A, Julfiqar. Giant cell tumor of the first metatarsal. J Cancer Res Ther 2011;7:208-10. [Google Scholar]
- 5.Palmerini E, Picci P, Reichardt P, Downey G. Malignancy in giant cell tumor of bone: A review of the literature. Technol Cancer Res Treat 2019;18:1533033819840000. [Google Scholar]
- 6.Su YP, Chen WM, Chen TH. Giant-cell tumors of bone: An analysis of 87 cases. Int Orthop 2004;28:239-43. [Google Scholar]
- 7.Gitelis S, Mallin BA, Piasecki P, Turner F. Intralesional excision compared with en bloc resection for giant-cell tumors of bone. J Bone Joint Surg Am 1993;75:1648-55. [Google Scholar]
- 8.Frassica FJ, Sim FH, Pritchard DJ, Chao EY. Subchondral replacement: A comparative analysis of reconstruction with methyl methacrylate or autogenous bone graft. Chir Organi Mov 1990;75:189-90. [Google Scholar]
- 9.Puri A, Agarwal M. Treatment of giant cell tumor of bone: Current concepts. Indian J Orthop 2007;41:101-8. [Google Scholar]
- 10.O’Connor PJ, Gibbon WW, Stone M, Mangham DC, Freeman SJ. Sonographic demonstration of fluid-fluid levels in an aneurysmal bone cyst secondary to a giant cell tumour of the calcaneus. Clin Radiol Extra 2004;59:43-7. [Google Scholar]
- 11.Turcotte RE, Wunder JS, Isler MH, Bell RS, Schachar N, Masri BA, et al. Giant cell tumor of long bone: A Canadian sarcoma group study. Clin Orthop Relat Res 2002;397:248-58. [Google Scholar]
- 12.Cribb GL, Cool P, Hill SO, Mangham DC. Distal tibial giant cell tumour treated with curettage and stabilisation with an Ilizarov frame. Foot Ankle Surg 2009;15:28-32. [Google Scholar]
- 13.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am 1987;69:106-14. [Google Scholar]
- 14.Garg N, Tanveer N, More S, Chauhan S. Giant cell tumor of clavicle in an elderly: Rare presentation of a common tumor with review of literature; 1980-2020. J Microsc Ultrastruct 2022;10:93-5. [Google Scholar]
- 15.Errani C, Ruggieri P, Asenzio MA, Toscano A, Colangeli S, Rimondi E, et al. Giant cell tumor of the extremity: A review of 349 cases from a single institution. Cancer Treat Rev 2010;36:1-7. [Google Scholar]
- 16.National Cancer Center. Musculoskeletal Tumor Committee JOA: Bone Tumor Registry in Japan. Tokyo: National Cancer Center; 2012. [Google Scholar]
- 17.Baptista AM, Camargo AF, Caiero MT, Rebolledo DC, Correia LF, de Camargo OP. GCT: What happened after 10 years of curettage and cement? Retrospective study of 46 cases. Acta Ortop Bras 2014;22:308-11. [Google Scholar]
- 18.Wood VE. The results of total claviculectomy. Clin Orthop Relat Res 1986;207:186-90. [Google Scholar]
- 19.Carrasco CH, Murray JA. Giant cell tumors. Orthop Clin North Am 1989;20:395-405. [Google Scholar]
- 20.Agrawal AC, Choudhary R, Verma S. The successful management of a repetitively infected recurrent proximal humerus giant cell tumour of 20 years’ duration with two-staged surgery: A rare case report. Cureus 2021;13:e14492. [Google Scholar]
- 21.Prosser GH, Baloch KG, Tillman RM, Carter SR, Grimer RJ. Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone? Clin Orthop Relat Res 2005;435:211-8. [Google Scholar]
- 22.Prosser GH, Baloch KG, Tillman RM, Carter SR, Grimer RJ. Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone? Clin Orthop Relat Res 2005;435:211-8. [Google Scholar]
- 23.Kivioja AH, Blomqvist C, Hietaniemi K, Trovik C, Walloe A, Bauer HC, et al. Cement is recommended in intralesional surgery of giant cell tumors: A Scandinavian Sarcoma Group study of 294 patients followed for a median time of 5 years. Acta Orthop 2008;79:86-93 [Google Scholar]
- 24.Arbeitsgemeinschaft Knochentumoren, Becker WT, Dohle J, Bernd L, Braun A, Cserhati M, et al. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am 2008;90:1060-7. [Google Scholar]
- 25.Bini SA, Gill K, Johnston JO. Giant cell tumor of bone. Curettage and cement reconstruction. Clin Orthop Relat Res 1995;321:245-50. [Google Scholar]
- 26.Wada T, Kaya M, Nagoya S, Kawaguchi S, Isu K, Yamashita T, et al. Complications associated with bone cementing for the treatment of giant cell tumors of bone. J Orthop Sci 2002;7:194-8. [Google Scholar]
- 27.Shih HN, Hsu RW, Sim FH. Excision curettage and allografting of giant cell tumor. World J Surg 1998;22:432-7. [Google Scholar]
- 28.Tharayil J, Patil RK. Salvage of foot with extensive giant cell tumour with transfer of vascularised fibular bone graft. Indian J Plast Surg 2011;44:150-6. [Google Scholar]
- 29.Leung KH, Lam AY, Ho KW, Shek TW. Giant cell tumor of the humeral head treated by denosumab: Implication to shoulder surgeons. Int J Shoulder Surg 2015;9:135-8. [Google Scholar]
- 30.Mak IW, Evaniew N, Popovic S, Tozer R, Ghert M. A translational study of the neoplastic cells of giant cell tumor of bone following neoadjuvant denosumab. J Bone Joint Surg Am 2014;96:e127. [Google Scholar]











