To effectively diagnose and treat infantile fibrosarcoma of the bone among the pediatric population, it is crucial to maintain a high level of suspicion for accurate diagnosis and to adopt a comprehensive, multidisciplinary treatment plan.
Dr. Said Saghieh, Department of Orthopaedic Surgery, American University of Beirut Medical Center, Cairo Street, Hamra, Beirut 1107 2020, Lebanon. E-mail: ss15@aub.edu.lb
Introduction: In adults, fibrosarcoma (FS) of the bone is a rare occurrence. Infantile FS, particularly in the distal radius, is an exceedingly uncommon tumor and, to the best of our knowledge, has not yet been documented in the literature. In the subsequent report, we present a case involving a 2-year-old male diagnosed with primary FS of the distal radius.
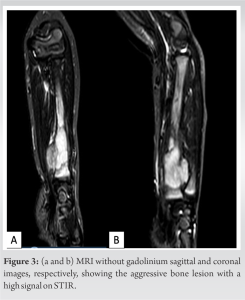
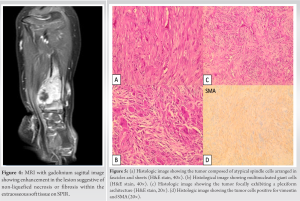
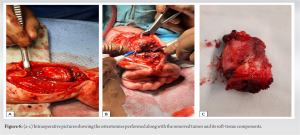
Case Report: We hereby report the case of a 2-year-old Caucasian boy presenting with primary bone FS in the distal radius. X-rays revealed an osseous mass with an extraosseous component. MRI showed heterogeneous enhancement, suggestive of non-liquefied necrosis or possible fibrosis within the extraosseous soft-tissue component. The patient underwent a resection of the tumor, followed by central translocation of the ulna.
Conclusion: Managing infantile FS of the bone requires a multidisciplinary approach. A high index of suspicion is crucial for diagnosing the tumor. Further studies are needed to enhance our approach and management of infantile FS of the bone.
Keywords: Fibrosarcoma, Infantile, Surgery.
In adults, fibrosarcoma (FS) of the bone is an infrequent entity, comprising 5% of primary bone tumors. Infantile FS of the distal radius is exceptionally rare and, to the best of our knowledge, has not yet been reported in the literature. In this case report, we discuss a 2-year-old male patient presenting with primary FS of the distal radius. We will also review the literature and current management strategies, with an emphasis on the importance of adopting a multidisciplinary approach.
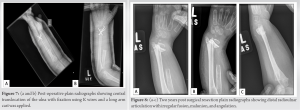
A 2-year-old male presented with a distal radius tumor on the left side that had been growing for 8 months. He presented with a pathological fracture, which was treated conservatively and healed 4 weeks after the injury. The mass had been noticed a couple of weeks before the fall but was not investigated until the patient came to the emergency department due to his fall. The family history was negative for consanguineous parents; however, it revealed that the mother had pre-eclampsia during her pregnancy. Upon physical examination, he had an obviously palpable, tender lesion with no erythema. He had no fever, no lymphadenopathy, and no upper respiratory symptoms. Basic laboratory investigations were within normal ranges, and the lactate dehydrogenase level was normal. Both oral and written informed consents were obtained from the legal guardians regarding the case study and future publications. X-ray imaging showed the osseous origin of the mass with an extraosseous component (Fig. 1a and b).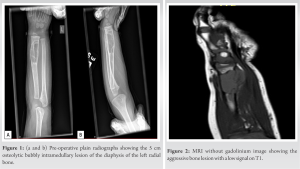

Primary fibrosarcoma (FS) is an infrequent entity, comprising less than 5% of all primary bone tumors. Adult-type bone FS, a rare and malignant tumor originating from fibroblasts, predominantly affects adults and, only on very rare occasions, children, exhibiting an inconsistent response to therapy [3, 4, 5]. Infantile fibrosarcoma (IFS) most commonly involves the distal extremities, followed by the head, neck, and trunk, while the adult type is more frequently found in the thigh [6, 7, 8]. IFS, a rare malignant mesenchymal neoplasm derived from fibroblasts, has an incidence rate of fewer than 0.2 cases per million in the pediatric age group [9, 10]. It primarily affects children within the first 2 years of life; however, it can be present at birth and can also develop during early childhood [9, 11]. The most commonly recommended treatment is surgical resection [7]. To the best of our knowledge, infantile primary FS of the bone has not yet been reported in the literature, and its management has not been thoroughly investigated. Maintaining a high index of suspicion is crucial when faced with an unorthodox presentation of an infantile bony tumor. Previous studies have reported several FS cases of the bone with various suggested management algorithms. However, the aforementioned reported cases did not include young children. Although instances of regression following chemotherapy—without the need for any surgical intervention—have been reported, surgical resection remains the most commonly recommended treatment [12]. To date, all reported cases of primary FS in bone have involved either adults or children above 7 years of age. In their 2021 paper, Kosemehmetoglu et al. reviewed all reported cases of primary FS of the bone, identifying a total of 9 cases. The patients had a mean age of 39 years, with ages ranging from 14 to 71 years, and consisted of 5 males and 4 females [13]. Additionally, a retrospective study by Papagelopoulos et al. investigated 92 cases of primary FS of the bone and discussed their outcomes. Patients who underwent adequate radical resection demonstrated a better prognosis than those treated with intralesional or marginal resection. The study emphasized that further investigations are needed to explore the role of perioperative chemotherapy in patients with FS, concluding that age >40, tumors in the axial skeleton, and high-grade tumors are pivotal risk factors that negatively impact overall survival [5]. However, none of the patients in the series was under 5 years old.
A multidisciplinary approach is essential for managing infantile FS of the bone, and maintaining a high index of suspicion is crucial for diagnosing this tumor in the pediatric population. Additional studies are warranted to enhance the approach and management of infantile FS of the bone.
Developing a comprehensive strategy that addresses multiple aspects is a must for an effective infantile FS of bone treatment. Furthermore, keeping a vigilant approach is necessary to accurately identify this tumor in children. Further studies are required to enhance our knowledge and improve the management of infantile FS of the bone.
References
- 1.Behjati S, Tarpey PS. What is next generation sequencing? Arch Dis Child Educ Pract Ed 2013;98:236-8. [Google Scholar]
- 2.Kumar KR, Cowley MJ, Davis RL. Next-generation sequencing and emerging technologies. Semin Thromb Hemost 2019;45:661-73. [Google Scholar]
- 3.Bridge J, Hogendoorn PC, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 4.Kilaikode S, Kuril S, Sedrak A, Sadanandan S. Sclerosing epithelioid fibrosarcoma of the parietal bone and adjacent meninges in an adolescent: A case report. World J Oncol 2013;4:255-7. [Google Scholar]
- 5.Papagelopoulos PJ, Galanis E, Frassica FJ, Sim FH, Larson DR, Wold LE. Primary fibrosarcoma of bone. Outcome after primary surgical treatment. Clin Orthop Relat Res 2000;373:88-103. [Google Scholar]
- 6.Chung EB, Enzinger FM. Infantile fibrosarcoma. Cancer 1976;38:729-39. [Google Scholar]
- 7.Himori K, Hatori M, Watanabe M, Moriya T, Ogose A, Hashimoto H, et al. Infantile fibrosarcoma of thigh--a case report. Ups J Med Sci 2005;110:85-93. [Google Scholar]
- 8.Soule EH, Pritchard DJ. Fibrosarcoma in infants and children: A review of 110 cases. Cancer 1977;40:1711-21. [Google Scholar]
- 9.Priya M, Singh P, Malhotra M, Angral S, Varshney S, Bhardwaj A, et al. Cervical infantile fibrosarcoma: A rare cause of paediatric parapharyngeal neck mass. Autops Case Rep 2020;10:e2020189. [Google Scholar]
- 10.Thebaud E, Mezel A, Leroy X, Orbach D. Fibrosarcoma in children and adolescents: Different entities for the same name. Bull Cancer 2012;99:715-22. [Google Scholar]
- 11.Coffin CM, Jaszcz W, O’Shea PA, Dehner LP. So-called congenital-infantile fibrosarcoma: Does it exist and what is it? Pediatr Pathol 1994;14:133-50. [Google Scholar]
- 12.Sait SF, Danzer E, Ramirez D, LaQuaglia MP, Paul M. Spontaneous regression in a patient with infantile fibrosarcoma. J Pediatr Hematol Oncol 2018;40:e253-5. [Google Scholar]
- 13.Kosemehmetoglu, K., Ardic F, Kilpatrick SE, Aydingoz U, Sumathi VP, Michal M. Sclerosing epithelioid fibrosarcoma of bone: morphological, immunophenotypical, and molecular findings of 9 cases. Virchows Arch, 2021. 478(4):767-777. [Google Scholar]









