Ochrobactrum anthropi is an emerging pathogen with an increase in the frequency of musculoskeletal infections that need vigilant monitoring to identify them early and treat them appropriately.
Dr. Madhan Jeyaraman, Department of Orthopaedics, Faculty of Medicine - Sri Lalithambigai Medical College and Hospital, Chennai, Tamil Nadu, India. E-mail: madhanjeyaraman@gmail.com
Introduction:Ochrobactrum anthropi is an opportunistic and rare human pathogen, which is seen widely in the environment. O. anthropi infections have been reported in both immunocompetent and immunocompromised individuals. There is no proper consensus on the diagnosis and management of O. anthropi related infections.
Case Report:We report a case of O. anthropi related left shoulder pyomyositis with distal clavicular and acromion osteomyelitis in an immunocompetent individual with an elaborative diagnostic and treatment algorithm for its effective management.
Conclusion:A comprehensive management strategy with a combination of implant removal (if present) with extensive surgical debridement of bone and soft tissue and intravenous antibiotics results in successful eradication of O. anthropi infection.
Keywords:Ochrobactrum anthropi, pyomyositis, osteomyelitis, immunocompetent, opportunistic infection.
Ochrobactrum anthropi is a Gram-negative and non-fermentative rare human pathogen, ubiquitously seen in the environment [1, 2]. Being an opportunistic bacterium, it is noted for bacteremia, localized infections, and catheter or stent-associated infections in both immunocompetent and immunocompromised individuals [3, 4, 5]. Reports have been made on its diagnostic challenges and difficulty in identifying the optimal therapeutic algorithm [6, 7, 8, 9]. Till date, there are no standard guidelines to diagnose and treat O. anthropi related infections [10, 11, 12]. We report a case of pyomyositis of the left shoulder with distal clavicular and acromion osteomyelitis due to O. anthropi and discuss our diagnostic and treatment algorithm for its effective management.
A 37-year-old male presented to the outpatient department with a history of fever, pain, and restriction of movements over the left shoulder, and generalized body pain for 20 days duration. Fever was high grade, intermittent with chills and rigor. The left shoulder pain was insidious in onset, gradually progressive, non-radiating, aggravated on left shoulder movements, without any relieving factors. On examination, the left shoulder was warm and tender with severe restriction of movements especially abduction and external rotation. Complete blood count showed leukocytosis with raised erythrocyte sedimentation rate and C-reactive protein levels. The inflammatory markers such as IL-1, IL-6, and procalcitonin were also raised. The blood culture was sent.
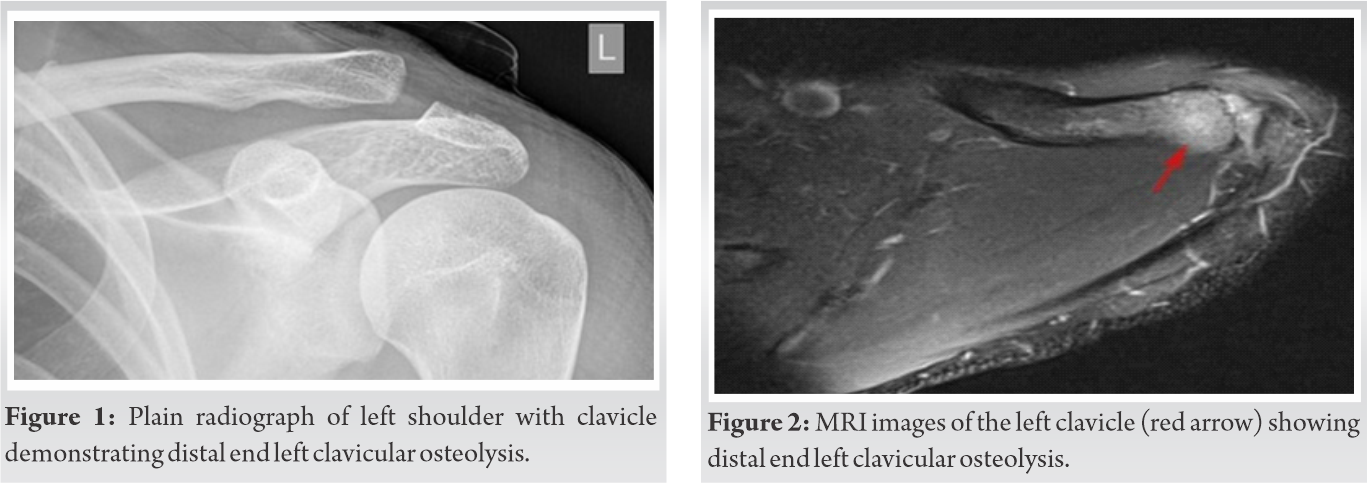
A plain radiograph of the left shoulder revealed no abnormality. The patient was started empirically on intravenous cefuroxime twice daily but he continued to have a persistent high-grade fever. MRI of left shoulder demonstrated ill-defined diffuse edema with peripheral enhancing collection involving all the rotator cuff muscles and marrow edema with a patchy enhancement of distal end of clavicle and acromion suggestive of osteomyelitis with pyomyositis and abscess (Fig. 1, 2).
Under general anesthesia, the pus was drained out and sent for culture and Gene Xpert MTB/RIF. The debrided tissue was also sent for biopsy which turned out to be pyomyositis. Blood and pus culture demonstrated O. anthropi showing susceptibility to cotrimoxazole, imipenem, meropenem, piperacillin-tazobactam, and resistance to aminoglycosides, cephalosporins, and fluoroquinolones. Gene Xpert was negative. The patient was started intravenous meropenem 1 gm thrice daily for 2 weeks followed by tablet co-trimoxazole (160 mg/800 mg) twice daily for 4 weeks. The patient was also supplemented with oral risedronate 35 mg once weekly for 12 weeks, oral calcium 500 mg once daily for 12 weeks, and Vit D3 60k IU once weekly for 12 weeks. The patient was motivated for an active range of shoulder movements and shoulder physiotherapy. The patient had an uneventful recovery and followed up for 12 months.
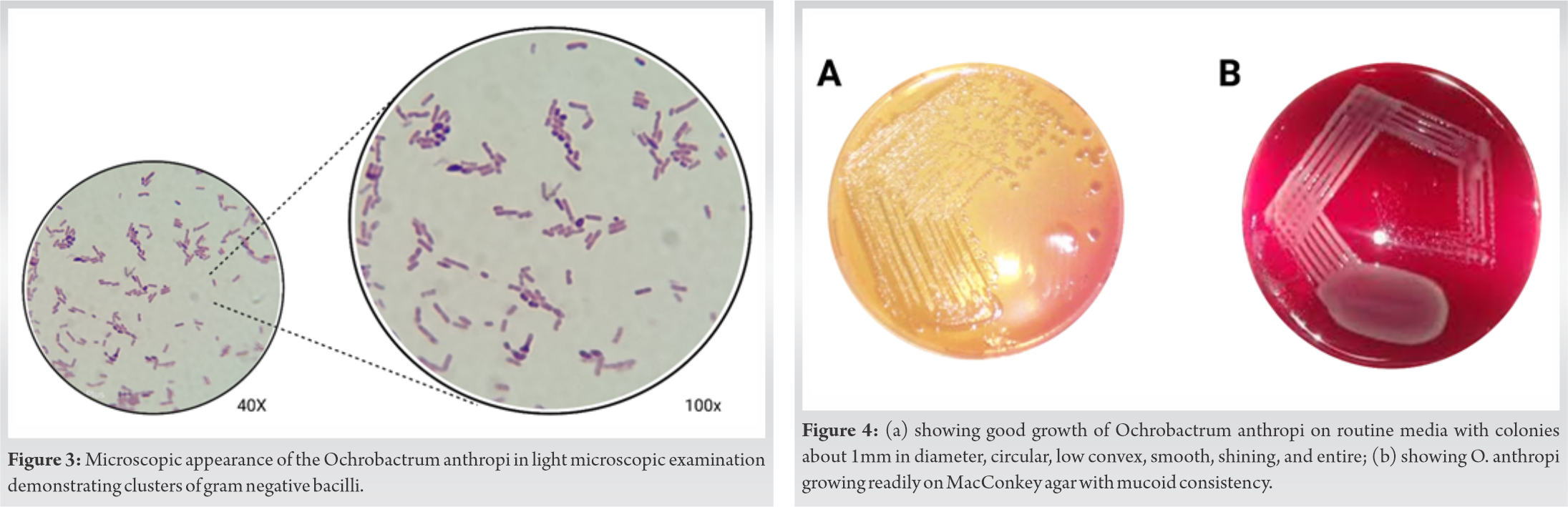
In 1988, Holmes et al. described the genus Ochrobactrum and its subtype species O. anthropi [13]. O. anthropi is recognized as belonging to the new genus Ochrobactrum, which was also called Brucella anthropi and Achromobacter that occasionally causes human infection [14]. The genus Ochrobactrum was named after the Greek word “Ochros” meaning yellow color [2]. The species name anthropi was given after being recovered from contaminated biologic products, hospital environments, intravascular cannulas, indwelling catheters, and clinical specimens such as blood, urine, feces, pus, CSF, wounds, bile, throat, and vagina [15, 16]. O. anthropi is an opportunistic, low virulent aerobic, non-fermentative, motile, oxidase-positive, indole-negative, gram-negative mesophilic bacillus as shown in (Fig. 3) [10]. API 20NE automated system misidentify O. anthropi as Brucella species because of its close relation [8, 14]. Hence, a confirmation is required with negative serum Brucella species antibodies in a patient with severe infections caused by O. anthropi bacteremia without any proper primary focus of infection and refractory to standard treatment [3].
O. anthropi is the name given to the urease-positive Achromobacter species previously designated as per CDC Vd-1, Vd-2 as Achromobacter spp. Biotypes 1 and 2 and Achromobacter Groups A, C, and D [10]. Ochrobactrum species are closely related to Brucella species with Ochrobactrum intermedium occupying a phylogenetic position that is intermediate between O. anthropi and Brucella [10, 17, 18]. They are oxidase-positive, saccharolytic, and motile with peritrichous flagella [10]. O. anthropi is ubiquitous and widely distributed in soil, plants, and water sources like normal saline, antiseptics, dialysis fluids, and swimming pools and also has been isolated from the hospital environment and contaminated foreign bodies such as intravascular catheters, graft tissues, and clinical specimens [19, 20, 21].
For O. anthropi, good growth is observed on routine media in 24 h. Colonies are about 1mm in diameter and appear circular, low convex, smooth, shining, and entire as shown in (Fig. 4) [22]. Isolates of O. anthropi have shown growth readily on MacConkey agar which appears mucoid consistency and are non-lactose fermenters as shown in (Fig. 4) [23]. Key tests useful in distinguishing O. anthropi from related organisms include their ability to hydrolyze urea, inability to hydrolyze esculin, and a negative ONPG test [2]. There are no biochemical tests currently available that separate O. intrermedium from O. anthropi [24]; however, it has been suggested that colistin (polymyxin E) and polymyxin B susceptibility can be used to O. intermedium is resistant and O. anthropi is sensitive [25].
In 1980, Appelbaum and Campbell described the first human clinical case with O. anthropi in a pancreatic abscess [26]. Infrequently, the clinical isolates of O. anthropi have been reported in humans secondary to nosocomial infections and hosts with immune dysregulation [27]. The risk factors for O. anthropi clinical disease spectrum are immune dysregulation, prolonged antibiotic therapy, allograft implantation, preceding trauma, and indwelling medical devices [20, 25]. Literature evidence demonstrates that O. anthropi is an emerging microbe with increased frequency in causing musculoskeletal infections either through direct inoculation from the environment or through hematogenous dissemination [16, 28, 29, 30].
Alnor et al. explained the biofilm formation with O. anthropi due to its capability to adhere to foreign bodies (i.e., silicon tubes) [23]. A few studies have shown the presence of O. anthropi in clinical specimens of intravascular cannulas and catheters [27, 31], biliary drainage tubes [32], chest tubes [32], and contaminated dural graft [33]. Till date, two reports were mentioned without any risk factor for O. anthropi infection in the musculoskeletal system in the literature and the present case is the third such case [19, 28]. In the musculoskeletal system, O. anthropi causes osteomyelitis, septic arthritis, osteochondritis, and soft tissue abscess [3, 4, 5, 6, 12, 13, 14, 15]. The review of O. anthropi infection in the musculoskeletal system is tabulated in (Table 1).
Types of specimen: Blood, wound swab, aspiration fluids, urine, nasal and nasopharyngeal swab, aural swab, CSF, stool, and central line catheter for culture.
Specimen processing: All samples must be processed as early as possible within 30 min to 1 h to avoid contamination.
Direct detection and cultures: The clinical samples are processed for culture and direct Gram stain which are visualized as slender, short to long gram-negative bacilli. Specimen suspected for O. anthropi can be cultured in 5% sheep blood agar, chocolate, and MacConkey agar. This organism also grows well in the broth of blood culture systems like brain heart infusion, nutrient broth, and thioglycolate broth. Colonies are small about 1mm in diameter and appear circular, low convex, smooth, shining, and entire resembling Enterobacteriaceae. Isolates of O. anthropi show mucoid appearance on MacConkey agar and are non-lactose fermenters whereas the Brucella group of organisms shows no growth in MacConkey agar [6, 8].
A few reports have been published in the literature stating the misidentification of Brucella group of organisms due to low homology with O. anthropi [8, 14, 37, 38]. Gopalsamy et al. reported misidentification of O. anthropi in Brucellosis infection as both the organisms are close phylogenetic relatives which result in cross-reactivity on Western blot and 16s ribosomal RNA sequence signatures [8]. Due to the overlap between two groups of organisms, the diagnosis becomes a great challenge. The American Society of Microbiology recommends that Brucella can be identified by growth patterns whereas O. anthropi can be identified by automated culture systems such as VITEK-2 and MALDI-TOF assay [39, 40].
Literature evidence for the management of O. anthropi is very limited. Though O. anthropi is a biofilm producer, it is difficult to eradicate the infection rather than the radical tissue debridement. There was no consensus available in terms of mono- or dual- or combination treatment modality for the eradication of O. anthropi infections. O. anthropi is resistant to penicillins and cephalosporins but usually susceptible to imipenem as it is consistent with inducible AmpC β-lactamase expression. The treatment should follow antibiotic susceptibility testing. Teyssier et al. demonstrated in vitro antibiotic susceptibility testing of 21 strains of O. anthropi which are susceptible to aminoglycosides, fluoroquinolones, netilmicin, colistin, and co-trimoxazole [41] but clinical failures were observed with ciprofloxacin [4] and imipenem [42] despite in vitro susceptibility. Yu et al. stated that monotherapy with an aminoglycoside or appropriate β-lactam antibiotics yielded a good clinical response in O. anthropi bacteremia [43]. The combination of implant removal (vascular catheters, dural grafts) with extensive surgical debridement of bone and soft tissue and intravenous antibiotics has to be given for the successful eradication of O. anthropi infections.
O. anthropi is an emerging pathogen with an increase in the frequency of musculoskeletal infections. This review provides a bird-eye view of the low virulent, opportunistic and nosocomial microbe in both immunocompetent and immunocompromised individuals and helps in reaching a diagnosis and providing appropriate management to curb the infection.
Vigilant monitoring and culturing of the infective specimen help in the diagnosis of O. anthropi. A comprehensive management strategy with a combination of implant removal (if present) with extensive surgical debridement of bone and soft tissue and intravenous antibiotics results in successful eradication of O. anthropi infection.
References
- 1.Mudshingkar SS, Choure AC, Palewar MS, Dohe VB, Kagal AS. Ochrobactrum anthropi: An unusual pathogen: Are we missing them? Indian J Med Microbiol 2013;31:306-8. [Google Scholar]
- 2.Hagiya H, Ohnishi K, Maki M, Watanabe N, Murase T. clinical characteristics of Ochrobactrum anthropi bacteremia. J Clin Microbiol 2013;51:1330. [Google Scholar]
- 3.Aguilera-Arreola MG, Ostria-Hernández ML, Albarrán-Fernández E, Juárez-Enriquez SR, Majalca-Martínez C, Rico-Verdín B, et al. Correct identification of Ochrobactrum anthropi from blood culture using 16rRNA sequencing: A first case report in an immunocompromised patient in Mexico. Front Med 2018;5:205. [Google Scholar]
- 4.Daxboeck F, Zitta S, Assadian O, Krause R, Wenisch C, Kovarik J. Ochrobactrum anthropi bloodstream infection complicating hemodialysis. Am J Kidney Dis 2002;40:E17. [Google Scholar]
- 5.Gómez MP, Esteban AM, Daza JA, Nieto JA, Alvarez D, García PP. Prosthetic mitral valve endocarditis due to Ochrobactrum anthropi: Case report. J Clin Microbiol 2004;42:3371-3. [Google Scholar]
- 6.Kämpfer P, Citron DM, Goldstein EJ, Scholz HC. Difficulty in the identification and differentiation of clinically relevant Ochrobactrum species. J Med Microbiol 2007;56 Pt 11:1571-3. [Google Scholar]
- 7.Scholz HC, Al Dahouk S, Tomaso H, Neubauer H, Witte A, Schloter M, et al. Genetic diversity and phylogenetic relationships of bacteria belonging to the Ochrobactrum-Brucella group by recA and 16S rRNA gene-based comparative sequence analysis. Syst Appl Microbiol 2008;31:1-16. [Google Scholar]
- 8.Gopalsamy SN, Ramakrishnan A, Shariff MM, Gabel J, Brennan S, Drenzek C, et al. Brucellosis initially misidentified as Ochrobactrum anthropi bacteremia: A case report and review of the literature. Open Forum Infect Dis 2021;8:ofab473. [Google Scholar]
- 9.Levy CE, Marson FA, Filho ÉB. Challenges in the identification of Ochrobactrum anthropi in blood and sputum cultures of patients with cystic fibrosis. Rev Epidemiol E Controle Infecção 2018;8:189-91. [Google Scholar]
- 10.Ryan MP, Pembroke JT. The Genus Ochrobactrum as major opportunistic pathogens. Microorganisms 2020;8:1797. [Google Scholar]
- 11.Thoma B, Straube E, Scholz HC, Al Dahouk S, Zöller L, Pfeffer M, et al. Identification and antimicrobial susceptibilities of Ochrobactrum spp. Int J Med Microbiol 2009;299:209-20. [Google Scholar]
- 12.Shanthini T, Manohar P, Samna S, Srividya R, Bozdogan B, Rameshpathy M, et al. Emergence of plasmid-borne bla oxa-181 gene in Ochrobactrum intermedium: First report from India. Access Microbiol 2019;1:e000024. [Google Scholar]
- 13.Holmes B, Popoff M, Kiredjian M, Kersters KY. Ochrobactrum anthropi Gen. Nov., sp. Nov. from human clinical specimens and previously known as group VD. Int J Syst Evol Microbiol 1988;38:406-16. [Google Scholar]
- 14.Trêpa J, Mendes P, Gonçalves R, Chaves C, Brás AM, Mesa A, et al. Brucella vertebral osteomyelitis misidentified as an Ochrobactrum anthropi infection. IDCases 2018;11:74-6. [Google Scholar]
- 15.Ashraf F. A case of Ochrobactrum anthropi-induced septic shock and infective endocarditis. R I Med J (2013) 2016;99:27-8. [Google Scholar]
- 16.Saveli CC, Levi M, Koeppe J. Ochrobactrum anthropi septic arthritis: Case report and implications in orthopedic infections. Infect Dis Rep 2010;2:e2. [Google Scholar]
- 17.Leclercq SO, Cloeckaert A, Zygmunt MS. Taxonomic organization of the family brucellaceae based on a phylogenomic approach. Front Microbiol 2020;10:3083. [Google Scholar]
- 18.Ashford RT, Muchowski J, Koylass M, Scholz HC, Whatmore AM. Application of whole genome sequencing and pan-family multi-locus sequence analysis to characterize relationships within the family brucellaceae. Front Microbiol 2020;11:1329. [Google Scholar]
- 19.Gigi R, Flusser G, Kadar A, Salai M, Elias S. Ochrobactrum anthropi-caused osteomyelitis in the foot mimicking a bone tumor: Case report and review of the literature. J Foot Ankle Surg 2017;56:851-3. [Google Scholar]
- 20.Wi YM, Sohn KM, Rhee JY, Oh WS, Peck KR, Lee NY, et al. Spontaneous bacterial peritonitis due to Ochrobactrum anthropi: A case report. J Korean Med Sci 2007;22:377-9. [Google Scholar]
- 21.Rastogi N, Mathur P. Ochrobactrum anthropi: An emerging pathogen causing meningitis with sepsis in a neurotrauma patient. J Infect Dev Ctries 2017;11:733-5. [Google Scholar]
- 22.Velasco J, Romero C, López-Goñi I, Leiva J, Díaz R, Moriyón I. Evaluation of the relatedness of Brucella spp. and Ochrobactrum anthropi and description of Ochrobactrum intermedium sp. nov., a new species with a closer relationship to Brucella spp. Int J Syst Bacteriol 1998;48 Pt 3:759-68. [Google Scholar]
- 23.Alnor D, Frimodt-Møller N, Espersen F, Frederiksen W. Infections with the unusual human pathogens Agrobacterium species and Ochrobactrum anthropi. Clin Infect Dis 1994;18:914-20. [Google Scholar]
- 24.Möller LV, Arends JP, Harmsen HJ, Talens A, Terpstra P, Slooff MJ. Ochrobactrum intermedium infection after liver transplantation. J Clin Microbiol 1999;37:241-4. [Google Scholar]
- 25.Kulkarni G, Gohil K, Misra V, Kakrani AL, Misra SP, Patole M, et al. Multilocus sequence typing of Ochrobactrum spp. isolated from gastric niche. J Infect Public Health 2017;10:201-10. [Google Scholar]
- 26.Appelbaum PC, Campbell DB. Pancreatic abscess associated with Achromobacter group Vd biovar 1. J Clin Microbiol 1980;12:282-3. [Google Scholar]
- 27.Kettaneh A, Weill FX, Poilane I, Fain O, Thomas M, Herrmann JL, et al. Septic shock caused by Ochrobactrum anthropi in an otherwise healthy host. J Clin Microbiol 2003;41:1339-41. [Google Scholar]
- 28.Wheen L, Taylor S, Godfrey K. Vertebral osteomylitis due to Ochrobactrum anthropi. Intern Med J 2002;32:426-8. [Google Scholar]
- 29.Jelveh N, Cunha BA. Ochrobactrum anthropi bacteremia. Heart Lung J Crit Care 1999;28:145-6. [Google Scholar]
- 30.Battaglia TC. Ochrobactrum anthropi septic arthritis of the acromioclavicular joint in an immunocompetent 17 year old. Orthopedics 2008;31:606. [Google Scholar]
- 31.Caroleo B, Malandrino P, Liberto A, Condorelli D, Patanè F, Maiese A, et al. Catheter-related bloodstream infections: A root cause analysis in a series of simultaneous Ochrobactrum anthropi infections. Curr Pharm Biotechnol 2019;20:609-14. [Google Scholar]
- 32.Cieslak TJ, Drabick CJ, Robb ML. Pyogenic infections due to Ochrobactrum anthropi. Clin Infect Dis 1996;22:845-7. [Google Scholar]
- 33.Christenson JC, Pavia AT, Seskin K, Brockmeyer D, Korgenski EK, Jenkins E, et al. Meningitis due to Ochrobactrum anthropi: An emerging nosocomial pathogen. A report of 3 cases. Pediatr Neurosurg 1997;27:218-21. [Google Scholar]
- 34.Barson WJ, Cromer BA, Marcon MJ. Puncture wound osteochondritis of the foot caused by CDC group Vd. J Clin Microbiol 1987;25:2014-6. [Google Scholar]
- 35.Luo JM, Guo L, Chen H, Yang PF, Xiong R, Peng Y, et al. A study of pre-operative presence of micro-organisms in affected knee joints of rheumatoid arthritis patients who need total knee arthroplasty. Knee 2017;24:409-18. [Google Scholar]
- 36.Bratschi C, Ly T, Weber A, Meuli-Simmen C, Conen A, Mauler F. Ochrobactrum anthropi infection of the hand. J Hand Surg Glob Online 2020;2:365-7. [Google Scholar]
- 37.Elsaghir AA, James EA. Misidentification of Brucella melitensis as Ochrobactrum anthropi by API 20NE. J Med Microbiol 2003;52 Pt 5:441-2. [Google Scholar]
- 38.Poonawala H, Marrs Conner T, Peaper DR. The brief case: Misidentification of Brucella melitensis as Ochrobactrum anthropi by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). J Clin Microbiol 2018;56:e00914-17. [Google Scholar]
- 39.Ushakova TK, Lerin AIP, Pareja JS, Aspas RD, Barcelona MP, García AS, et al. Identificación de Brucella melitensis como Ochrobactrum anthropi mediante MALDI-TOF MS. Rev Esp Quimioter 2020;33:223-4. [Google Scholar]
- 40.Gilligan PH, York MK. Sentinel Level Clinical Laboratory Guidelines for Suspected Agents of Bioterrorism and Emerging Infectious Diseases Brucella Species; 2016. Available from: https://asm.org/ASM/media/Policy-and-Advocacy/LRN/Sentinel%20Files/Brucella-2016-March.pdf [Last accessed on 2021 Nov 16]. [Google Scholar]
- 41.Teyssier C, Marchandin H, Jean-Pierre H, Diego I, Darbas H, Jeannot JL, et al. Molecular and phenotypic features for identification of the opportunistic pathogens Ochrobactrum spp. J Med Microbiol 2005;54 Pt 10:945-53. [Google Scholar]
- 42.Kern WV, Oethinger M, Kaufhold A, Rozdzinski E, Marre R. Ochrobactrum anthropi bacteremia: Report of four cases and short review. Infection 1993;21:306-10. [Google Scholar]
- 43.Yu WL, Lin CW, Wang DY. Clinical and microbiologic characteristics of Ochrobactrum anthropi bacteremia. J Formos Med Assoc Taiwan Yi Zhi 1998;97:106-12. [Google Scholar]