Our instituional treatment protocol of size and location based treatment, use of allografts and extramedullary implant options have shown promising primary results both in terms of radiology and functional outcomes in Paediatric Monostotic Fibrous Dysplasia of proximal femur.
Dr. Soham Banik, Department of Orthopaedics, Government Medical College, Nagpur, Maharashtra, India. E-mail: sohambanik.mbbs@gmail.com
IntroductionMonostotic fibrous dysplasia is a rare genetic non-inherited orthopedic condition presenting at any age with variable presentation. Proximal femur being the most common site, the mechanical factor predisposes it to increased chances of pathological fracture, which makes it challenging to choose an appropriate treatment modality and implant selection in children.
Case PresentationIn this retrospective case series, six children aged 7–12 years with monostotic fibrous dysplasia with or without fracture were treated with different treatment modalities from 2015 to 2020. Extended curettage and bone grafting and stabilization were done with extramedullary implants such as locking plates and DHS. Autograft alone or combination with allograft was chosen according to size of lesion. Patients without fracture were treated with curettage and artificial bone graft substitute with hip spica. Patients were followed up to 12 months. Revised Musculoskeletal Tumor Society (MSTS) score at each follow-up and Toronto Extremity Salvage Score (TESS) at final follow-up.
ResultsMean fracture healing time was 14.8 ± 2.28 weeks. in patients with fracture. Full weight-bearing was started at average 15.67 ± 2.94 weeks. One patient had shortening of 1 cm but none had any surgical site infection, loss of correction, or varus collapse more than 5°. Mean revised MSTS at 12 months was 24.2 ± 2.28 in patients with curettage, bone grafting, and internal fixation, while it was 27 in patients with curettage, grafting with hip spica. Mean TESS was found to be 90 ± 9.41 in internal fixation group, while it was 95 in patient with curettage, grafting with hip spica.
ConclusionTreatment modality should be chosen wisely after analyzing pre-operative radiograph, lesion size, presence of pathological fracture, and each patient profile. Extramedullary fixation devices can be an alternate choice of implant in children and adolescents for monostotic fibrous dysplasia. Long duration follow-up and patient counseling should be done for recurrence of lesion and deformities.
KeywordsMonostotic fibrous dysplasia, pediatric pathological fracture, extramedullary device, allograft, autograft.
Fibrous dysplasia is a benign intramedullary disease usually present in childhood, that is, monostotic or polyostotic form, with the former being the most common form ranging from 75% to 80% [1]. It is not a true bone tumor, rather a developmental disorder with the proliferation of primitive fibrous tissue within the medullary canal, which on expansion causes thinning of the cortex [2]. True incidence and prevalence of fibrous dysplasia are difficult to estimate; though not rare, it accounts for 5–7% of all benign bone lesions presentation at adolescence and early adulthood without any gender predilection [3]. The mean age of presentation for monostotic type is 20 years, whereas polyostotic type presents earlier than 10 years [4]. Lytic lesions can affect various skeletal structures, with the proximal femur being the most common, followed by tibia, humerus, rib, clavicle, and craniofacial skeleton in decreasing order [5]. Monostotic forms are less aggressive than the polyostotic type, which seizes expansion after skeletal maturity. Sometimes, there can be an incidental finding or remain concealed up to late adulthood. The later form can remain active after skeletal maturity and may be associated with endocrinopathies and café-au-lait spots (McCune Albright Syndrome) or myxomas (Mazabraud syndrome) [3, 6]. Among the varied clinical spectrum pain is the first and most common presenting symptom, patients are sometimes unaware of the lesion and present with pathological fracture [5, 7]. The other clinical features may be swelling, limb length discrepancy, and deformity. Osteolytic lesions in the weight-bearing region increase the risk of pathological fracture. Mechanical demand on the proximal femur makes it the anatomic site with the highest risk for developing a pathological fracture, leading to nonunion and avascular necrosis [8]. Hence, aggressive management of these fractures should be done. Various treatment modalities of such fractures have been described, such as conservative with bisphosphonate therapy, curettage, and strut grafting, IM nailing, and plate-screw fixation [9]. In the literature, IM nails are described to be superior to plate-screw fixation [10, 11]. However, finding appropriately sized nails in pediatric patients is difficult. There is a paucity of literature in use of bone graft with extramedullary implants and its functional outcome in fibrous dysplasia of pediatric femur. There seems to have knowledge gap in the ideal treatment for fibrous dysplasia in pediatric patients as well as there remains controversy regarding the use of type of bone graft with or without fixation device. We treated six pediatric patients with monostotic fibrous dysplasia with different treatment modalities according to the size and type of lesion in our tertiary care center. We evaluated their functional outcome using the revised musculoskeletal tumor society scoring system (MSTS) [12] and TESS [13].
A retrospective case series conducted in tertiary care hospital in Central India after the institutional review board’s permission. From 2015 to 2020, six patients aged 7–12 years presented to us with monostotic fibrous dysplasia with chief complaints of pain at the hip and thigh, who were treated with different surgical modalities and followed up for at least 12 months.
Preoperative evaluation
AP and lateral radiographs of pelvis with both hips and thighs were taken. All the patients underwent a skeletal survey. Polyostotic lesion patients were excluded from the study. The femurs were classified radiologically according to the classification by Zhang et al. [14]. At least one of the following features had to be present to be considered as fibrous dysplasia (a) grayish ground-glass appearance, (b) endosteal scalloping, (c) shepherd crook deformity, (d) intramedullary expansile lesion with a smooth sclerotic margin, and (e) rind sign and parrot beak sign [14, 15, 16]. A pre-operative CT scan was done to evaluate (a) the location and size of the lesion, (b) extension of fracture line, and (c) reduced bone strength (thickness of remaining cortical bone <50% of original thickness on axial CT). As radiological features were highly suggestive of a benign pathology like fibrous dysplasia, pre-operative histological confirmation was not done in our study. However, post-operative histological study was done and confirmed features suggestive of fibrous dysplasia in all cases.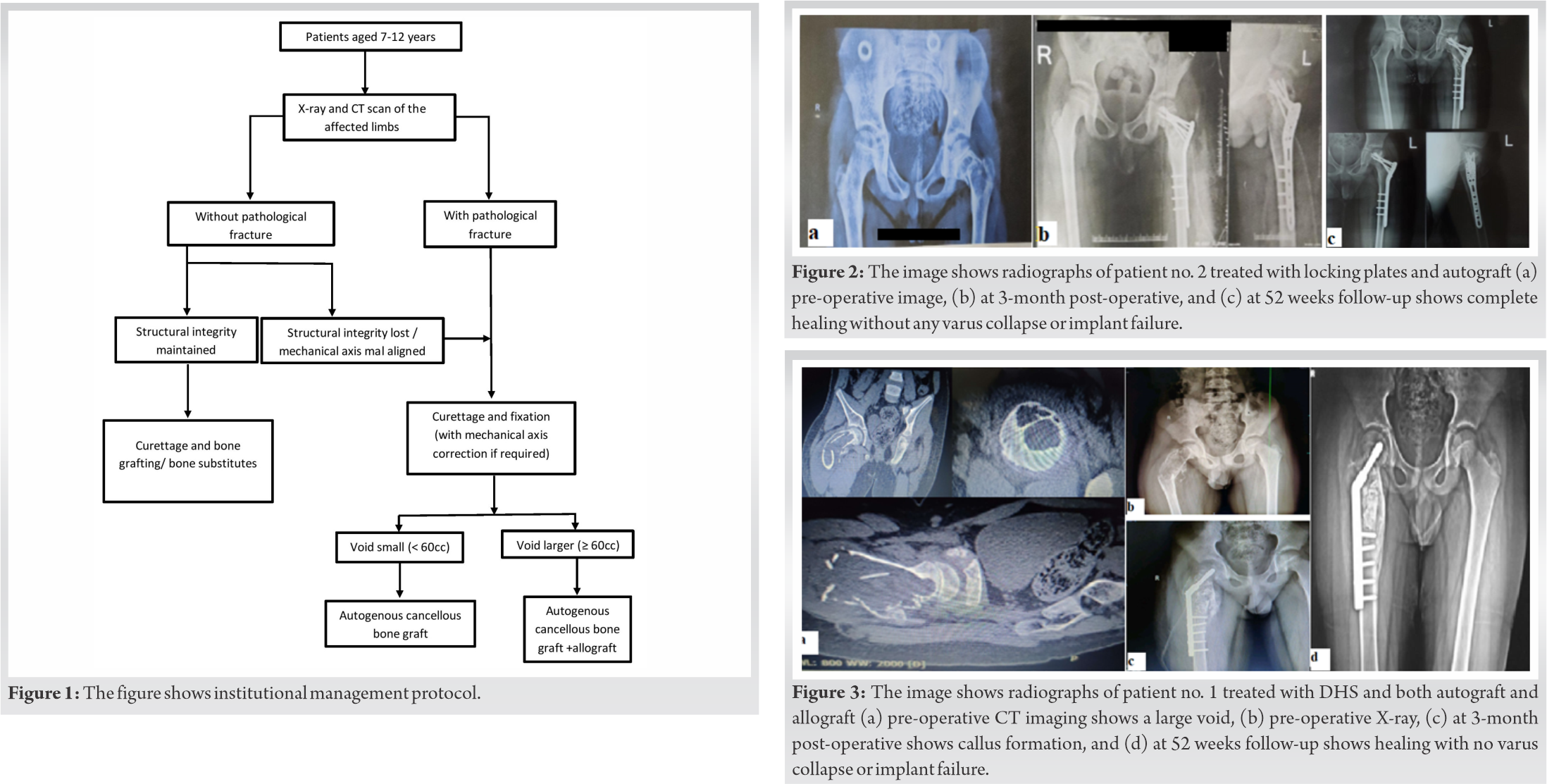
Surgical modalities
Two patients came to us with moderate to severe pain, limping, and difficulty in weight-bearing. Radiographs and CT scan showed that they had a Type 2 lesion with typical intramedullary location and ground-glass appearance suggestive of fibrous dysplasia [14]. They were advised curettage with bone substitute like G-BONE (synthetic granules of calcium hydroxyapatite in crystalline form) and post-operative hip spica cast for 8 weeks and then were given a hip orthosis. One of them (patient number 3; Table 1) was not willing for surgery and started on conservative measures like protected weight-bearing and calcium Vitamin D supplements. After 2 months, this patient returned with an insufficiency fracture at the femoral neck and was taken for curettage and bone grafting along with internal fixation with dynamic hip screw. Four patients had a history of trivial trauma and presented with pathological fracture of proximal femur with lytic lesion. One of them had a previous history of pain around the hip, which was relieved after taking NSAIDs. Rest four patients did not have any previous significant history.
Patients with pathological fracture were placed in skin traction and medically optimized for surgery. Surgical plan was formulated and individualized for each patient with aim of (a) local control of disease by extended curettage with phenol and sending tissue for histopathological examination, (b) filling of void with bone graft/graft substitutes, (c) neutralization of fracture with extramedullary implants, and (d) correction of deformity of neck or proximal femur and achieving mechanical alignment. We chose a surgical plan according to the institutional protocol (Fig. 1). Intraoperative curettage was done, and tissues were sent for histopathologic confirmation followed by extended curettage using 85% phenol and then washing it with 96% ethanol [17] followed by bone grafting. An appropriate extramedullary implant was selected, as we did not have access to growing nails. Two patients were fixed with dynamic hip screw and two with locking plates (one PHILOS plate and one distal femur locking plate) according to availability of implants. To get a better hold with the implant, we had to cross the physis in all of our patients. Small cavities (<60 cc) were filled up with autogenous cancellous iliac bone graft [18] (Fig. 2a-c). The larger cavities were filled with autogenous cancellous graft and then supplemented with freeze-dried allogenic cancellous bone graft after 20 min of rehydration with normal saline (Fig. 3a-d). Operative time, blood loss, and any other complications were recorded.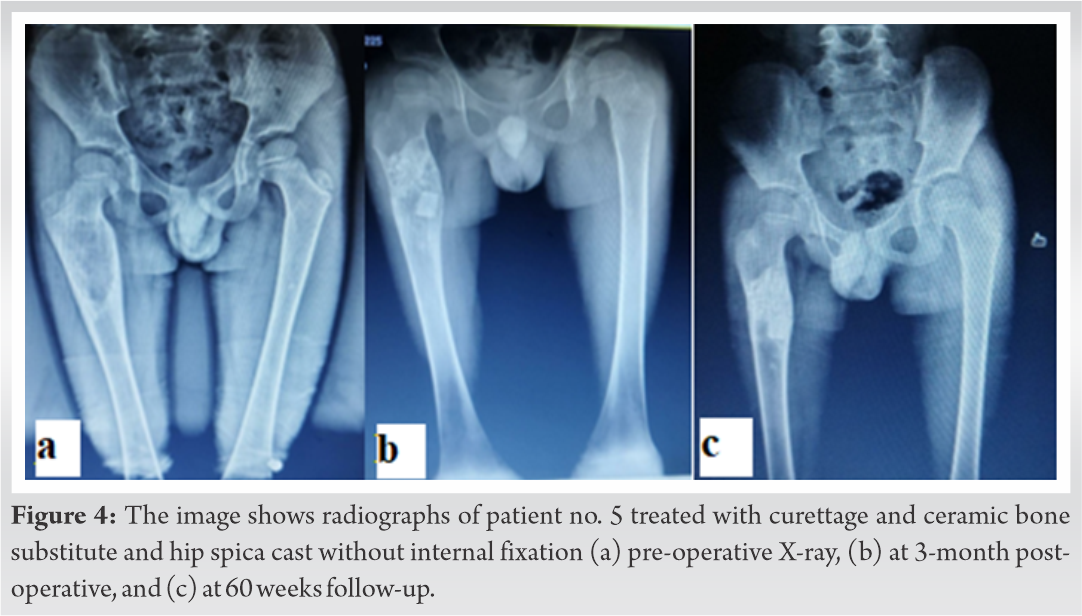
After care
Postoperatively, the patient without internal fixation (patient number 5) was kept on hip spica cast for 8 weeks and then cast was removed and started on partial weight-bearing till radiological signs of healing (Fig. 4a-c).
For patients with pathological fracture and internal fixation, knee range of motion, ankle pumps, static quadriceps strengthening, and bedside mobilization were started immediately. After check dress, patients were discharged on an average of the 5th post-operative day with hip orthosis. Suture removal was done on the 15th post-operative day. Passive hip adduction and abduction were started after suture removal. Passive straight leg rising test (SLRT) followed by active-assisted SLRT was started at 3 weeks. Active SLRT was started at 4 weeks. After achieving satisfactory hip and knee ROM, patients were mobilized non-weight-bearing with walker and hip orthosis. Patients were kept non-weight-bearing for at least 12 weeks or radiological signs of healing whichever are earlier. Full weight-bearing was started after radiological confirmation for adequate callus formation. As we had to get better hold in the neck, we had to cross physis during internal fixation, we counseled the patient regarding limb length discrepancy.
Follow-up
All six patients were followed up at 1, 3, 6, and 12 months. At each follow-up, each patient was evaluated radiologically for fracture union, mechanical axis alignment and implant position, any varus collapse and clinically for pain, range of motion, shortening, development of any deformity, gait, and patient compliance toward the treatment protocol. All the data were noted and produced as revised MSTS scores. At the final follow-up of each patient, mean 54.33 ± 3.2 weeks (range, 52–60 weeks), TESS was calculated for the patient using the pre-determined questionnaires.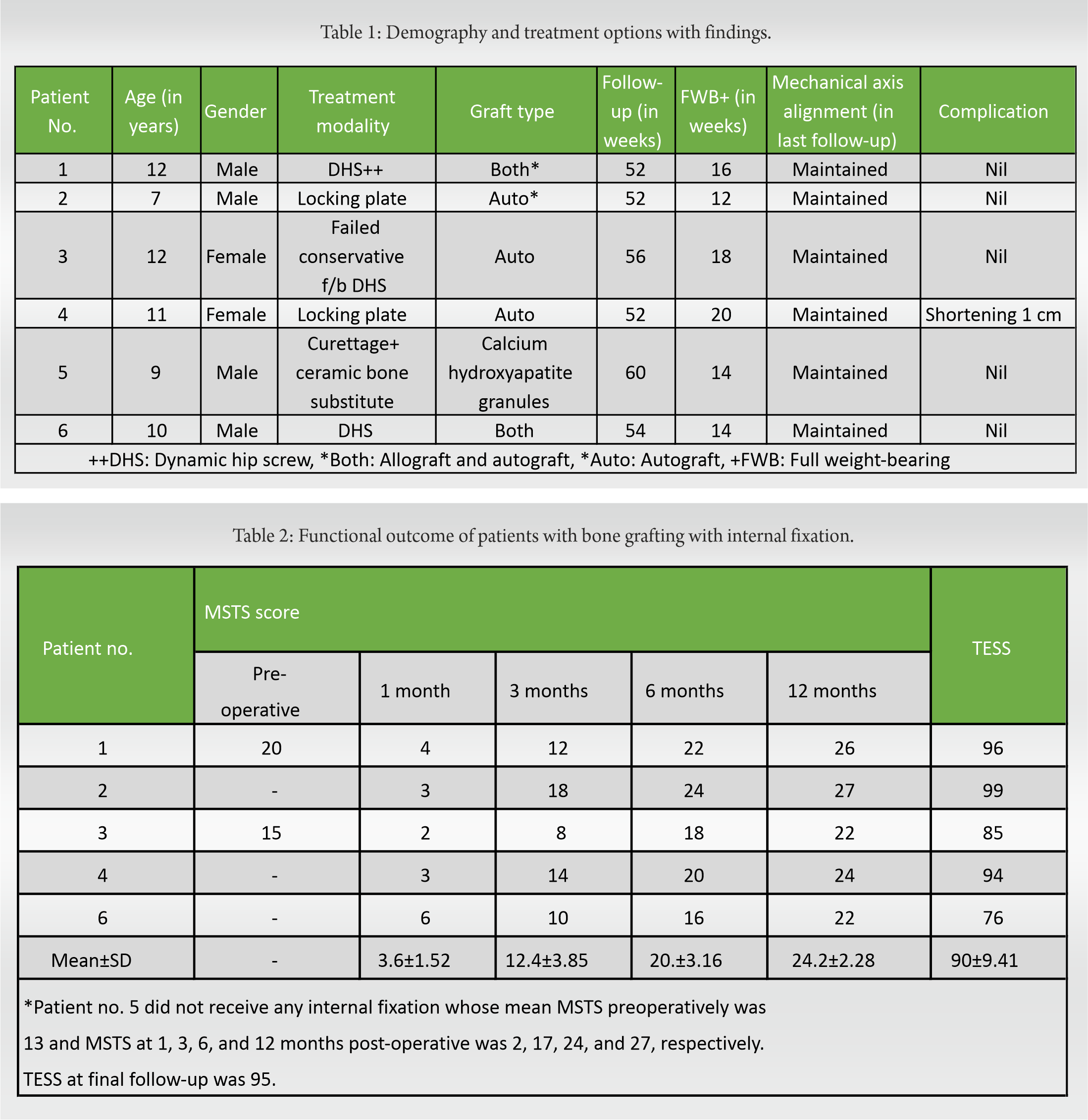
Each patient was given a serial number according to the chronologic order of presentation (Table 1). Patient nos. 1, 2, 4, and 6 presented with pathological fracture. Patient 3 had failed conservative therapy, presented with insufficiency fracture at 2 months follow-up, and was later internally fixed with grafting. Patient no. 5 was treated with simple curettage and calcium hydroxyapatite granules. In the patients with pathological fracture, the mean surgical duration was 112.29 ± 20.25 min. In the non-fracture patient, surgical time was found to be 100 min. The average blood loss during surgery was 201.43 ± 33.88 ml. The mean follow-up of all the patients was 54.33 ± 3.2 weeks. Patient no. 5 with artificial bone graft (calcium hydroxyapatite granules) showed healing at 14 weeks. Patient nos. 1 and 6 received both autograft and allograft, and fracture site healing was seen at 14 and 16 weeks, respectively. Patient nos. 2, 3, and 4 received only autograft and healing seen at 12, 14, and 18 weeks, respectively. In our study, mean fracture healing was seen at 14.8 ± 2.28 weeks. However, our study sample is small to reach a definitive conclusion about the effects of various grafts on healing time. Full weight-bearing was started in all the patients at an average of 15.67 ± 2.94 weeks. None of the patient had any surgical site infection. However, one patient (pt. no. 4) had shortening of 1 cm. None had significant varus collapse (>5°) at fracture site. The intraoperative tissue curettage histopathology report supported our diagnosis. Histopathological reports showed woven structure of bone lacking osteoblastic rimming, Sharpey’s fibers along trabeculae edges, and characteristic “alphabet soup” appearance. During follow-up visits, the functional outcome was studied using revised MSTS score and TESS for every patient (Table 2).
Fibrous dysplasia of bone is a genetic, non-inherited orthopedic condition resulting from the activating mutation of the GNAS1 gene. However, fibrous dysplasia rarely undergoes malignant transformation [5, 7]. Most of the studies in the literature have shown adult patients presenting with pathological fracture, though from our study, it can be said that pathological fractures can occur in children and adolescent group which needs special attention. Most of the lesions are small and silent or with minimal symptoms such as pain, difficulty walking, and swelling. Some are large lesions with moderate-to-severe symptoms such as severe pain, limp, deformity, and limb length discrepancy. Some may present with a fracture with trivial trauma and diagnosed with pathological fracture during fracture films [18].
Treatment of the lesions located at this site makes the treatment difficult. Increased stress concentration and muscle pull proportionately increase the chances of fracture at the proximal femur. The age of the patient, lesion size, presence of fracture, and behavior of the patient influence the treatment choice [19]. Various treatment modalities such as bisphosphonate therapy, curettage and bone grafting, internal fixation with nailing or plate-screw system, and valgus osteotomy have been described in the literature [11, 20, 21, 22]. Baghdadi and Arkader had described about “total bone fixation” with intramedullary fixation devices in Ippolito type 3 and 4 proximal femoral fibrous dysplasia and pre-operative 3D printing to determine accurate osteotomy sites in difficult cases of Shepherds crook deformity [23]. Various bone grafts are used in the treatment of these lesions. Ippolito et al. and Leet and Collins showed that cortical bone grafts are frequently resolved and do not hold disease progression [5, 24]. Glancy et al. showed no difference in success rates between allograft and autograft in lesions <60 ccs. Whereas lesions more than 60 ccs autografts appeared to be superior [25]. All the surgical patients in our study showed fracture site healing radiologically and clinically at around 14 weeks postoperatively. Follow-up radiographs showed increased cortical thickness, maintained neck-shaft angle, and no recurrence of lesion. Lala et al. showed that bisphosphonate therapy in children and adolescents with McCune Albright syndrome increased bone density and decreased pain [26]. Leet and Collins suggested that bisphosphonate does not change the natural history of the disease but relieves pain [24]. However, in all these studies, size or type of the lesion was not taken into consideration. Bhadada et al. in their case report described a novel therapy of injecting intralesional bisphosphonate, which at follow-up showed improved pain scores and decreased uptake on bone scintigraphy [27]. In our study, we avoided bisphosphonate therapy as most of our study population came with pathological fracture and underwent surgery. One of the two patients (patient number 3) with type 2 femur was started on conservative therapy but progressed into an insufficiency fracture after trivial trauma at medial calcar after 2 months of therapy and needed bone grafting and fixation. The other patient with a similar lesion was treated with curettage and ceramic hydroxyapatite bone substitute. Both patients showed radiological and clinical signs of union at an around 14 weeks postoperatively. However, in our study, the healing of the lesion cannot be attributed to the use of ceramic bone substitute in patient number 5. Very little literature assessed the functional outcome after treating monostotic fibrous dysplasia in the proximal femur in children using revised MSTS score and TESS. A study by Nishida et al. showed that all patients had improved MSTS scores at final follow-up after using cortical autograft and internal fixation [15]. A study by Ebeid et al. showed that mean MSTS was 27.63 when they used internal fixation without grafting [20]. Despite different treatment modalities, we found that improved MSTS score from 1-month post-operative period to final follow-up. At 1 month, the MSTS score decreased initially because of post-operative pain, weight-bearing restrictions, and emotional factors. However, the MSTS score gradually improved at subsequent follow-ups. Similarly, MSTS score and TESS of curettage and artificial grafting patient were 27 and 95, respectively, which are comparable with the patients with different treatment modalities in our study. The decreased MSTS score in these two patients during early follow-up was due to prolonged immobilization and delayed weight-bearing compared to other patients.
In this case series of six patients with monostotic fibrous dysplasia of proximal femur, we found that the ideal treatment modality should be chosen wisely and individualized after analyzing pre-operative radiograph, lesion size, presence of pathological fracture, and each patient profile. Furthermore, the type of graft should be decided preoperatively according to the size of the lesion. Extramedullary fixation devices can be an alternate choice of implant in children and adolescents for monostotic fibrous dysplasia. According to our study, it is crucial for the patients to follow the pre-determined treatment protocol for early and better functional outcomes.
Proximal femur monostotic fibrous dysplasia in children can be challenging due to lack of graft and intramedullary implant options. Our institutional treatment protocol of size and location-based treatment, use of allografts, and extramedullary implant options have shown promising primary results both in terms of radiology and functional outcomes.
References
- 1.1. Smith SE, Kransdorf MJ. Primary musculoskeletal tumors of fibrous origin. Semin Musculoskelet Radiol 2000;4:73-88. [Google Scholar]
- 2.2. Henry A. Monostotic fibrous dysplasia. J Bone Joint Surg Br 1969;51:300-6. [Google Scholar]
- 3.3. DiCaprio MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am 2005;87:1848-64. [Google Scholar]
- 4.4. Parekh SG, Donthineni-Rao R, Ricchetti E, Lackman RD. Fibrous dysplasia. J Am Acad Orthop Surg 2004;12:305-13. [Google Scholar]
- 5.5. Ippolito E, Bray EW, Corsi A, De Maio F, Exner UG, Robey PG, et al. Natural history and treatment of fibrous dysplasia of bone: A multicenter clinicopathologic study promoted by the European pediatric orthopaedic society. J Pediatr Orthop B 2003;12:155-77. [Google Scholar]
- 6.6. Wirth WA, Leavitt D, Enzinger FM. Multiple intramuscular myxomas. Another extraskeletal manifestation of fibrous dysplasia. Cancer 1971;27:1167-73. [Google Scholar]
- 7.7. Harris WH, Dudley HR, Barry RJ. The natural history of fibrous dysplasia. An orthopaedic, pathological, and roentgenographic study. J Bone Joint Surg Am 1962;44-A:207-33. [Google Scholar]
- 8.8. Carvallo PI, Griffin AM, Ferguson PC, Wunder JS. Salvage of the proximal femur following pathological fractures involving benign bone tumors. J Surg Oncol 2015;112:846-52. [Google Scholar]
- 9.9. Majoor BC, Leithner A, van de Sande MA, Appelman-Dijkstra NM, Hamdy NA, Dijkstra PD. Individualized approach to the surgical management of fibrous dysplasia of the proximal femur. Orphanet J Rare Dis 2018;13:72. [Google Scholar]
- 10.10. Stephenson RB, London MD, Hankin FM, Kaufer H. Fibrous dysplasia. An analysis of options for treatment. J Bone Joint Surg Am 1987;69:400-9. [Google Scholar]
- 11.11. Kushare IV, Colo D, Bakhshi H, Dormans JP. Fibrous dysplasia of the proximal femur: Surgical management options and outcomes. J Child Orthop 2014;8:505-11. [Google Scholar]
- 12.12. Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res 1993;286:241-6. [Google Scholar]
- 13.13. Davis AM, Wright JG, Williams JI, Bombardier C, Griffin A, Bell RS. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res 1996;5:508-16. [Google Scholar]
- 14.14. Zhang X, Chen C, Duan H, Tu C. Radiographic classification and treatment of fibrous dysplasia of the proximal femur: 227 Femurs with a mean follow-up of 6 years. J Orthop Surg Res 2015;10:171. [Google Scholar]
- 15.15. Nishida Y, Tsukushi S, Hosono K, Nakashima H, Yamada Y, Urakawa H, et al. Surgical treatment for fibrous dysplasia of femoral neck with mild but prolonged symptoms: A case series. J Orthop Surg Res 2015;10:63. [Google Scholar]
- 16.16. Han I, Choi ES, Kim HS. Monostotic fibrous dysplasia of the proximal femur: Natural history and predisposing factors for disease progression. Bone Joint J 2014;96:673-6. [Google Scholar]
- 17.17. Verdegaal SH, den Hartigh J, Hogendoorn PC, Brouwers HF, Taminiau AH. Phenol levels during intralesional curettage and local adjuvant treatment of benign and low-grade malignant bone tumours. Clin Sarcoma Res 2012;2:10. [Google Scholar]
- 18.18. Shih HN, Cheng CY, Chen YJ, Huang TJ, Hsu RW. Treatment of the femoral neck amd trochanteric benign lesions. Clin Orthop Relat Res 1996;328:220-6. [Google Scholar]
- 19.19. Tong Z, Zhang W, Jiao N, Wang K, Chen B, Yang T. Surgical treatment of fibrous dysplasia in the proximal femur. Exp Ther Med 2013;5:1355-8. [Google Scholar]
- 20.20. Ebeid WA, Hasan BZ, Mesregah MK. Management of fibrous dysplasia of proximal femur by internal fixation without grafting: A retrospective study of 19 patients. J Am Acad Orthop Surg Glob Res Rev 2018;2:e057. [Google Scholar]
- 21.21. Boyce AM, Kelly MH, Brillante BA, Kushner H, Wientroub S, Riminucci M, et al. A randomized, double blind, placebo-controlled trial of alendronate treatment for fibrous dysplasia of bone. J Clin Endocrinol Metab 2014;99:4133-40. [Google Scholar]
- 22.22. Nakashima Y, Kotoura Y, Nagashima T, Yamamuro T, Hamashima Y. Monostotic fibrous dysplasia in the femoral neck. A clinicopathologic study. Clin Orthop Relat Res 1984;191:242-8. [Google Scholar]
- 23.23. Baghdadi S, Arkader A. Fibrous Dysplasia: Recent Developments and Modern Management Alternatives. JPOSNA; 2020. Available from: https://www.jposna.org/ojs/index.php/jposna/article/view/84 [Last accessed on 2022 Apr 13]. [Google Scholar]
- 24.24. Leet AI, Collins MT. Current approach to fibrous dysplasia of bone and McCune-Albright syndrome. J Child Orthop 2007;1:3-17. [Google Scholar]
- 25.25. Glancy GL, Brugioni DJ, Eilert RE, Chang FM. Autograft versus allograft for benign lesions in children. Clin Orthop Relat Res 1991;262:28-33. [Google Scholar]
- 26.26. Lala R, Matarazzo P, Bertelloni S, Buzi F, Rigon F, de Sanctis C. Pamidronate treatment of bone fibrous dysplasia in nine children with McCune-Albright syndrome. Acta Paediatr 2000;89:188-93. [Google Scholar]
- 27.27. Bhadada SK, Pal R, Sood A, Dhiman V, Saini UC. Co-administration of systemic and intralesional zoledronic acid in a case of fibrous dysplasia: A potentially novel therapy. Front Endocrinol (Lausanne) 2019;10:803. [Google Scholar]








